本文作者:石油醚
概要
汤文军:(1974年浙江余姚)中科院上海有机化学研究所研究员、课题组长;同时兼任上海科技大学物质学院教授、博导和华东理工大学药学院博导。有机化学家,课题组主页:http://wenjuntang.sioc.ac.cn/index.html
经历
- 1991-1995年华东理工大学获得学士学位
- 1995-1998年上海有机化学研究所获得有机化学硕士学位(马大为院士)
- 1998-2003年宾夕法尼亚州立大学获得博士学位(张绪穆教授)
- 2003-2005年斯克利普斯研究所博士后(Professor K. C. Nicolaou)
- 2005-2009年美国Boehringer Ingelheim药业公司药物工艺部门(高级科学家)
- 2009-2011年美国Boehringer Ingelheim药业公司药物工艺部门(首席科学家)
- 2018-至今中科院上海有机化学研究所研究员、课题组长
- 上海科技大学物质学院教授、博导
- 华东理工大学药学院博导
获奖经历
- 2019 WuXi AppTech Life Science and Chemistry Award
- 2018 Young and Middle-Aged Leading Scientists, Engineers and Innovators
- 2017 The National Science Fund for Distinguished Young Scholars
- 2015 Asian Core Program Lectureship Award
- 2015 National Homogeneous Catalysis Youth Award
- 2014 May 4th Medal, The Science and Technology Commission of Shanghai Municipality
- 2014 Shanghai Science&Technology System Youth Award
- 2013 Shanghai “Pujiang Talents” Program
- 2012 Thieme Chemistry Journal Award
- 2010 Excellence in Action Award,2010, Boehringer Ingelheim Pharmaceuticals, Inc.
- 2009 President’s Award 2009, Boehringer Ingelheim Pharmaceuticals, Inc.
- 2009 Individual Excellence Award 2009, Boehringer Ingelheim Pharmaceuticals, Inc.
- 2008 Team Spirit Award (twice) 2008, Boehringer Ingelheim Pharmaceuticals, Inc.
- 2007 Golden Achievement Award,2007, Boehringer Ingelheim Pharmaceuticals, Inc.
- 2006 Golden Achievement Award, 2006, Boehringer Ingelheim Pharmaceuticals, Inc.
- 2005 Golden Achievement Award, 2005, Boehringer Ingelheim Pharmaceuticals, Inc.
- 2002 Dalalian Fellowship, 2002, The Pennsylvania State University
- 2001 Dalalian Fellowship, 2001, The Pennsylvania State Univeristy
- 2000 Dalalian Graduate Research Award, 2000, The Pennsylvania State University
研究方向
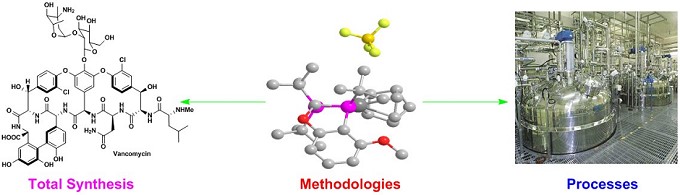
汤文军研究员课题组目前的研究内容主要是:
- 发展高效、实用、绿色的催化反应方法学以及开发用于催化反应高效配体和催化剂
- 复杂天然产物全合成
- 药物绿色合成
1. 发展高效、实用、绿色的催化反应方法学
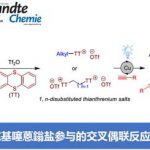
(a) 手性磷配体的设计与开发
手性磷配体在过渡金属催化的不对称反应中发挥着重要的作用。然而,在催化反应体系中仍然存在许多反应性和选择性等挑战性难题。而设计与合成含有特殊结构的新型手性磷配体作为高效的解决方案。汤文军研究小组已设计和发展了一系列具有显著结构特征的P-手性双膦/单磷配体1,2,并在大位阻偶联3-5、不对称偶联6-9、不对称环化9-12以及不对称氢化反应13-16中表现出优异的效率,同时也为复杂天然产物和药物的合成提供了一种实用、绿色且高效的催化方案。(图1)
图1 P-手性双膦/单磷配体(图片来自汤老师课题组主页)
(b) 亮点工作介绍
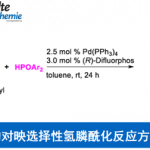
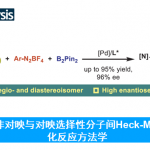
(1) 具有轴手性的联芳基化合物广泛存在于药物和天然产物分子中。通过不对称偶联合成具有轴手性的联芳基结构,尤其是手性邻位四取代联芳基化合物一直具有挑战性。。汤文军研究员通过开发一种新型P-手性单膦配体和设计新的反应模式,成功发展了用于高效合成手性邻位四取代联芳基化合物的不对称Suzuki-Miyaura偶联反应。基于此方法,合成了一系列邻位四取代联苯基和联萘基的独特结构。同时也实现了对棉酚的高效不对称全合成5。(图 2)(Yang, H.; Sun, J.; Gu, W.; Tang, W.* “Enantioselective Cross-Coupling for Axially Chiral Tetra-ortho-Substituted Biaryls and Asymmetric Synthesis of Gossypol”.J. Am. Chem. Soc. 2020, 142, 8036–8043)
图2 不对称Suzuki-Miyaura偶联反应(图来自acs)
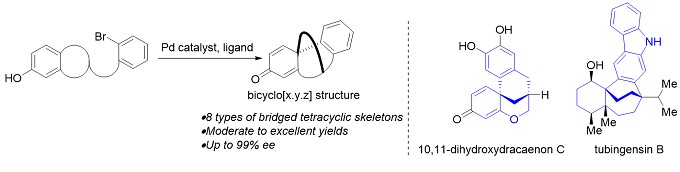
(2) 虽然在复杂多环天然产物的合成方面已取得了巨大的进步,但仍然缺乏有效且通用的方法来合成具有全碳四元中心桥联多环骨架的化合物。在此,汤文军研究员课题组报道了一种简便且通用的方法,通过钯催化的去芳化环化反应,可构建多种桥联多环骨架的衍生物。(图3)(Mu, X.; Yu, H.; Peng, H.; Xiong, W.; Wu, T.; Tang, W.* “Expedite Construction of Various Bridged Polycyclic Skeletons by Palladium-Catalyzed Dearomatization”.Angew. Chem., Int. Ed. 2020, 59, 8143-8147.)
图3 钯催化去芳化环化反应构建多种桥联多环骨架(图来自汤老师课题组主页)
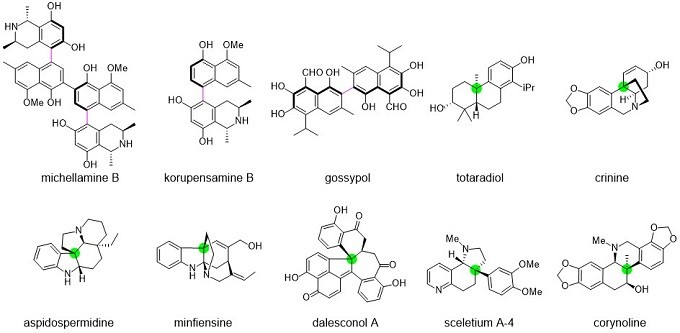
2. 天然产物的全合成5,9,17-19
汤文军研究员使用不对称的Suzuki-Miyaura交叉偶联、脱芳构环化和位阻α-芳基化作为关键步骤,以简洁高效的方式完成了一系列萜烯、类固醇、生物碱和聚酮等天然产物的不对称全合成。(图 4)
图 4天然产物全合成(图来自汤老师课题组主页)
其他
1. Chem-Station对汤文军研究员的“钯催化去芳化环化反应构建多种桥联多环骨架”的工作做了介绍。
图片来自chem-station
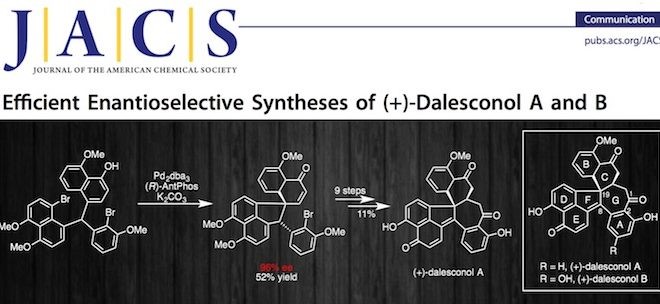
2. Chem-Station对汤文军研究员的“(+)-Dalesconol A和B的全合成”的工作做了介绍。
图来自chem-station
3. 汤文军研究员设计和发展了一系列有显著结构特征的P-手性双膦/单膦配体被收录在
“Li, K.; Tang, W.”Air-stable P-chiral Phosphorus Ligands for Asymmetric Catalysis and Synthesis” of Full Book “The Strem Chemiker”.Vol. XXXI No. 1 October, 2019”一书中。
图来自The Strem Chemiker
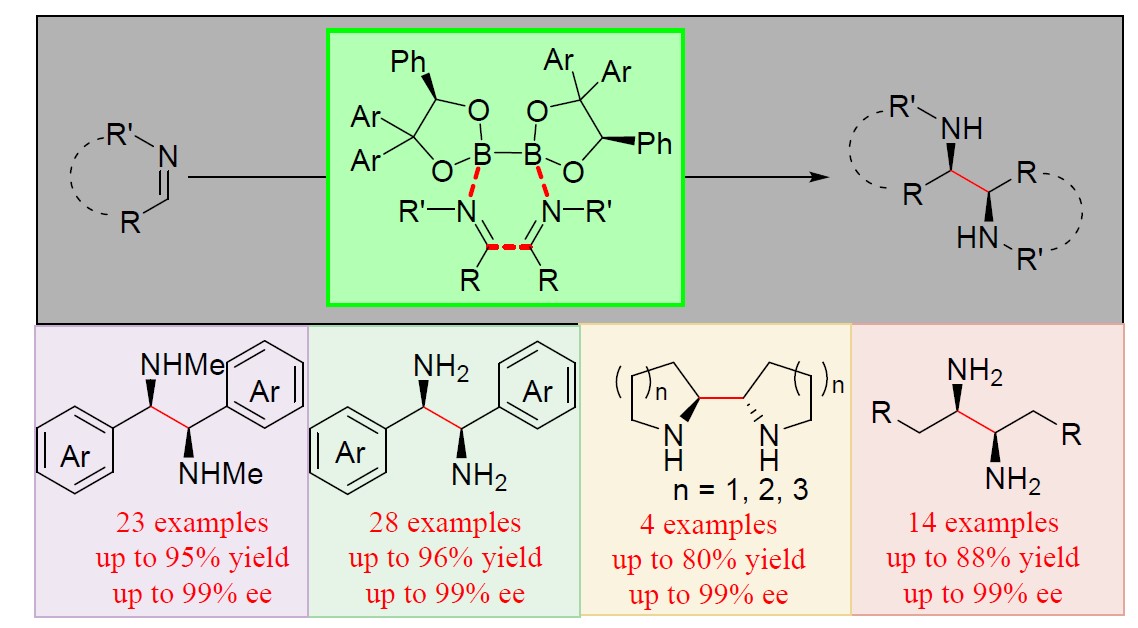
4. Chem-Station对汤文军研究员的“手性联硼酸酯促进亚胺的不对称还原偶联反应”的工作做了介绍。
参考文献
- [1] Xu, G., Senanayake, C. H. & Tang, W. P-Chiral Phosphorus Ligands Based on a 2,3-Dihydrobenzo[d][1,3]oxaphosphole Motif for Asymmetric Catalysis. Acc. Chem. Res. (2019) 52, 1101-1112, doi:10.1021/acs.accounts.9b00029.
- [2] Fu, W. & Tang, W. Chiral Monophosphorus Ligands for Asymmetric Catalytic Reactions. ACS Catal. (2016) 6, 4814-4858, doi:10.1021/acscatal.6b01001.
- [3] Tang, W. et al. A General and Special Catalyst for Suzuki–Miyaura Coupling Processes. Angew. Chem. Int. Ed. (2010) 49, 5879-5883, doi:10.1002/anie.201002404.
- [4] Xu, G., Fu, W., Liu, G., Senanayake, C. H. & Tang, W. Efficient Syntheses of Korupensamines A, B and Michellamine B by Asymmetric Suzuki-Miyaura Coupling Reactions. J. Am. Chem. Soc. (2014) 136, 570-573, doi:10.1021/ja409669r.
- [5] Yang, H., Sun, J., Gu, W. & Tang, W. Enantioselective Cross-Coupling for Axially Chiral Tetra-ortho-Substituted Biaryls and Asymmetric Synthesis of Gossypol. J. Am. Chem. Soc. (2020) 142, 8036-8043, doi:10.1021/jacs.0c02686.
- [6] Li, C., Chen, T., Li, B., Xiao, G. & Tang, W. Efficient Synthesis of Sterically Hindered Arenes Bearing Acyclic Secondary Alkyl Groups by Suzuki–Miyaura Cross-Couplings. Angew. Chem. Int. Ed. (2015) 54, 3792-3796, doi:10.1002/anie.201411518.
- [7] Huang, L. et al. Highly Enantioselective Rhodium-Catalyzed Addition of Arylboroxines to Simple Aryl Ketones: Efficient Synthesis of Escitalopram. Angew. Chem. Int. Ed. (2016) 55, 4527-4531, doi:10.1002/anie.201600979.
- [8] Li, B., Li, T., Aliyu, M. A., Li, Z. H. & Tang, W. Enantioselective Palladium-Catalyzed Cross-Coupling of α-Bromo Carboxamides and Aryl Boronic Acids. Angew. Chem. Int. Ed( 2019).58, 11355-11359, doi:10.1002/anie.201905174.
- [9] Rao, X. et al. Efficient Synthesis of (−)-Corynoline by Enantioselective Palladium-Catalyzed α-Arylation with Sterically Hindered Substrates. Angew. Chem. Int. Ed. (2018) .57, 12328-12332, doi:10.1002/anie.201807302.
- [10] Hu, N. et al. Synthesis of Chiral α-Amino Tertiary Boronic Esters by Enantioselective Hydroboration of α-Arylenamides. J. Am. Chem. Soc. (2015) 137, 6746-6749, doi:10.1021/jacs.5b03760.
- [11] Du, K. et al. Enantioselective Palladium-Catalyzed Dearomative Cyclization for the Efficient Synthesis of Terpenes and Steroids. Angew. Chem. Int. Ed. (2015) 54, 3033-3037, doi:10.1002/anie.201411817.
- [12] Mu, X. et al. Construction of Various Bridged Polycyclic Skeletons by Palladium-Catalyzed Dearomatization. Angew. Chem. Int. Ed. (2020) 59, 8143-8147, doi:10.1002/anie.202000953.
- [13] Zhou, M. et al. Enantioselective Reductive Coupling of Imines Templated by Chiral Diboron. J. Am. Chem. Soc.(2020)142, 10337-10342, doi:10.1021/jacs.0c04558.
- [14] Zhu, J. et al. Enantioselective Rhodium-Catalyzed Addition of Arylboroxines to N-Unprotected Ketimines: Efficient Synthesis of Cipargamin. Angew. Chem. Int. Ed. (2019) 58, 16119-16123, doi:10.1002/anie.201910008.
- [15] Li, C. et al. Stereoelectronic Effects in Ligand Design: Enantioselective Rhodium-Catalyzed Hydrogenation of Aliphatic Cyclic Tetrasubstituted Enamides and Concise Synthesis of (R)-Tofacitinib. Angew. Chem. Int. Ed. (2019) 58, 13573-13583, doi:10.1002/anie.201908089.
- [16] Fu, W., Nie, M., Wang, A., Cao, Z. & Tang, W. Highly Enantioselective Nickel-Catalyzed Intramolecular Reductive Cyclization of Alkynones. Angew. Chem. Int. Ed. (2015) 54, 2520-2524, doi:10.1002/anie.201410700.
- [17] Yang, H. & Tang, W. Efficient Enantioselective Syntheses of Chiral Natural Products Facilitated by Ligand Design. The Chemical Record (2020). 20, 23-40, doi:10.1002/tcr.201900003
- [18] Zhao, G., Xu, G., Qian, C. & Tang, W. Efficient Enantioselective Syntheses of (+)-Dalesconol A and B. J. Am. Chem. Soc. (2017) 139, 3360-3363, doi:10.1021/jacs.7b00783.
- [19] Du, K. et al. Efficient Syntheses of (−)-Crinine and (−)-Aspidospermidine, and the Formal Synthesis of (−)-Minfiensine by Enantioselective Intramolecular Dearomative Cyclization. Chem. Sci. (2017) 8, 6247-6256, doi:10.1039/C7SC01859B.
- [20] 汤文军老师课题组主页:http://wenjuntang.sioc.ac.cn/index.html
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!


























No comments yet.