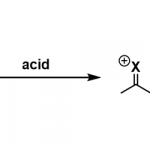
概要
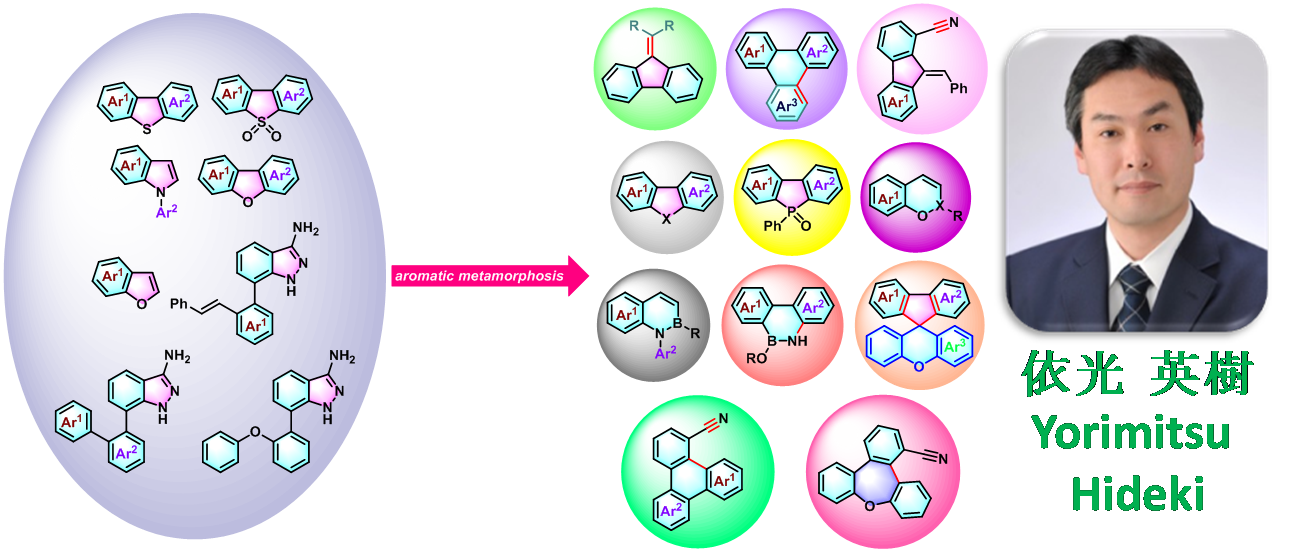
Yorimitsu aromatic metamorphosis反应 (Yorimitsu aromatic metamorphosis reaction)是将二苯并噻吩 (DBT, dibenzothiophene)[1]-[2]、二苯并噻吩二氧化物[3]-[5]、苯并呋喃[6]-[7]、二苯并呋喃[8]、吲哚[9]、3-氨基吲唑[10]等芳环与杂芳环骨架 (aromatic skeleton)通过一步或多步反应过程,转化为其它芳香与杂环芳香环系的反应。
这一全新的有机合成方法学概念由日本Kyoto大学理学院化学系(京都大学大学院理学研究科化学専攻,Department of Chemistry, Graduate School of Science, Kyoto University)的Yorimitsu (依光 英樹, Yorimitsu Hideki)研究室在2015年首次报道[1]。该方法学的建立,为各类重要的芳香化合物以及杂环芳香化合物的构建开辟了全新的方法[11]-[12]。
基本文献
- [1] D. Vasu, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2015, 54, 7162. doi: 10.1002/anie.201501992.
- [2] Hayate S., K. Nogi, H. Yormitsu, Chem. Lett. 2017, 46, 1122. doi: 10.1246/cl.170415.
- [3] M. Bhanuchandra, K. Murakami, D. Vasu, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2015, 54, 10234. doi: 10.1002/anie.201503671.
- [4] M. Bhanuchandra, H. Yorimitsu, A. Osuka, Org. Lett. 2016, 18, 384. doi: 10.1021/acs.orglett.5b03384.
- [5] M. Onoda, Y. Koyanagi, H. Saito, M. Bhanuchandra, Y. Matano, H. Yorimitsu, Asian J. Org. Chem. 2017, 6, 257. doi: 10.1002/ajoc.201600612.
- [6] S. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett. 2017, 19, 5557. doi: 10.1021/acs.orglett.7b02660.
- [7] H. Saito, S. Otsuka, K. Nogi, H. Yorimitsu, J. Am. Chem. Soc. 2016, 138, 15315. doi: 10.1021/jacs.6b10255.
- [8] Y. Kurata, S. Otsuka, N. Fukui, K. Nogi, H. Yorimitsu, A. Osuka, Org. Lett. 2017, 19, 1274. doi: 10.1021/acs.orglett.6b03861.
- [9] S. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett. 2019, 21, 3855. doi: 10.1021/acs.orglett.9b01353.
- [10] Y. Zhou, L. Lin,Y. Wang, J. Zhu, Q. Song, Org. Lett. 2019, 21, 7630. doi: 10.1021/acs.orglett.9b02933.
- [11] H. Yorimitsu, D. Vasu, M. Bhanuchandra, K. Murakami, A. Osuka, Synlett 2016, 27, 1765. doi: 10.1055/s-0035-1561617.
- [12] K. Nogi, H. Yorimitsu, Chem. Commun. 2017, 53, 4055. doi: 10.1039/C7CC00078B.
反应机理
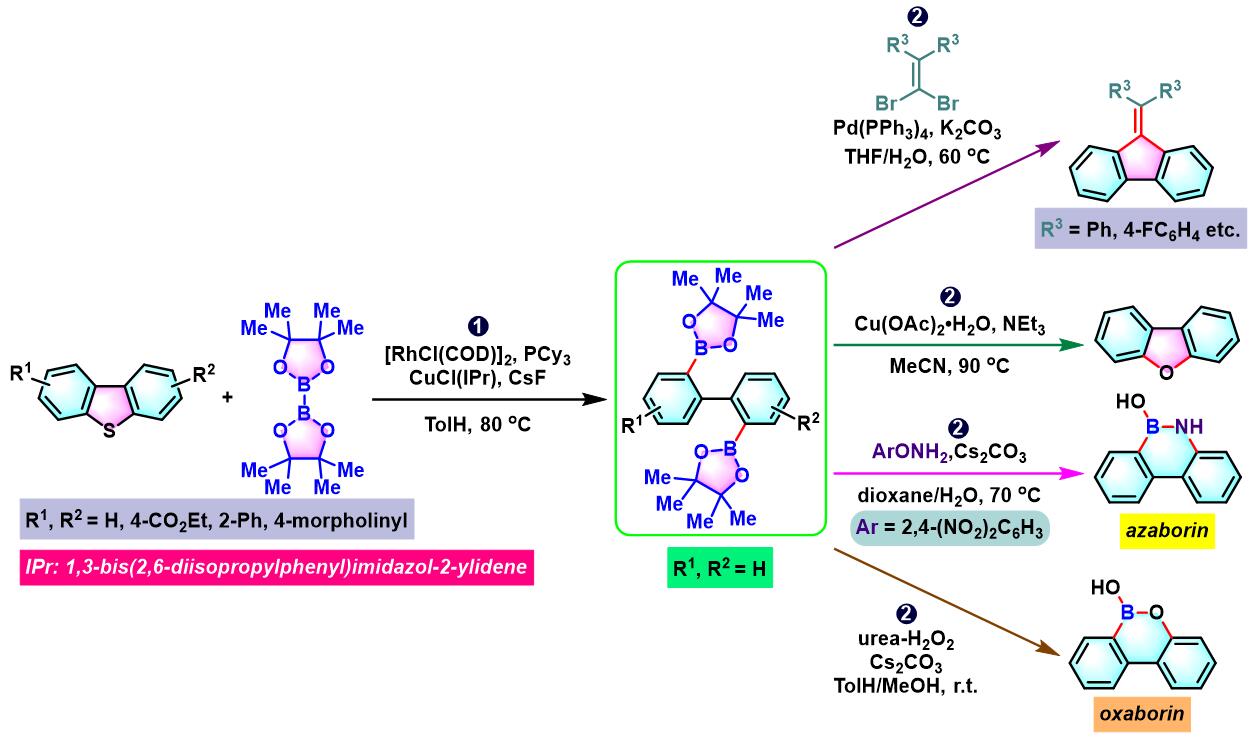
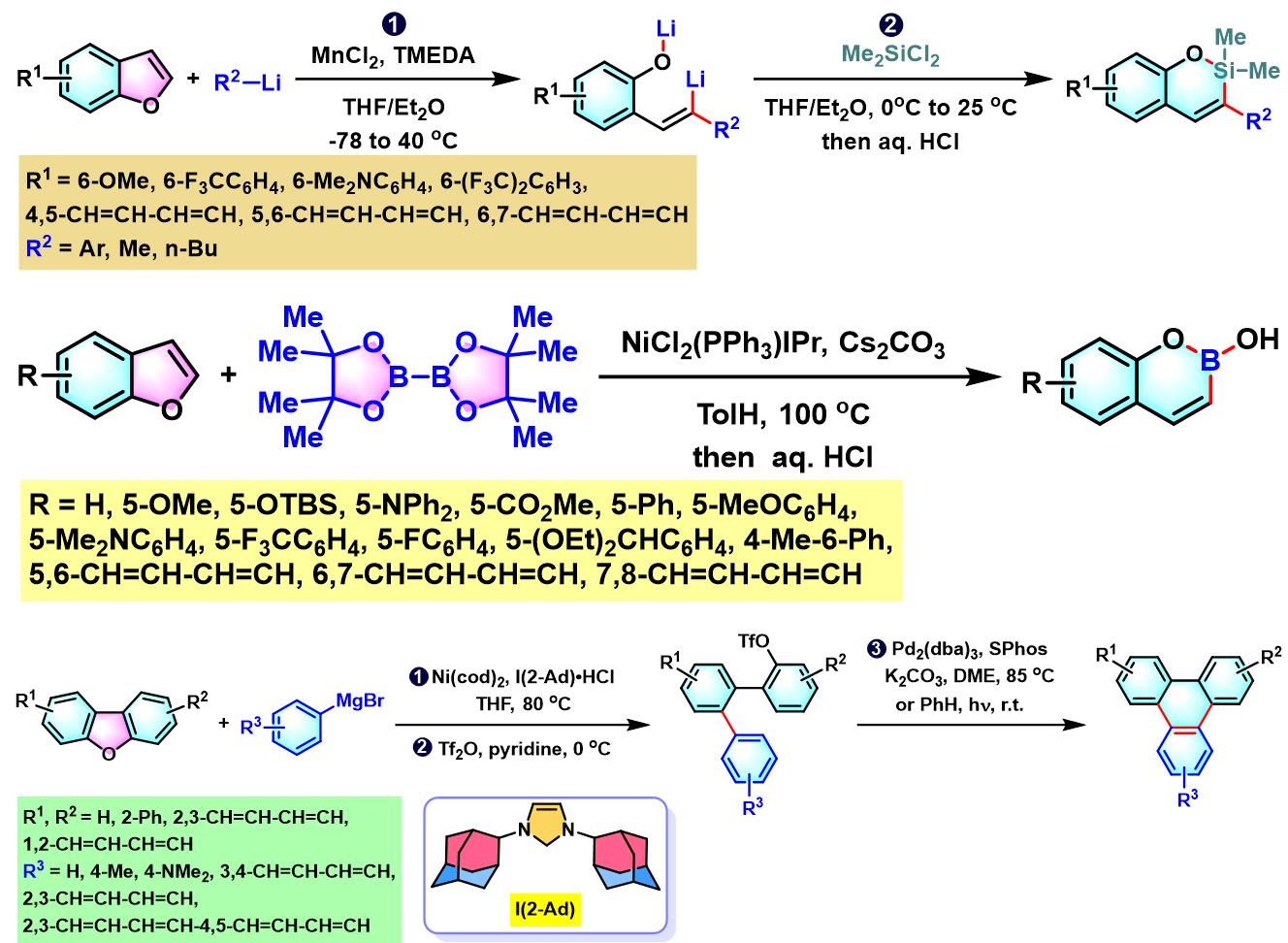
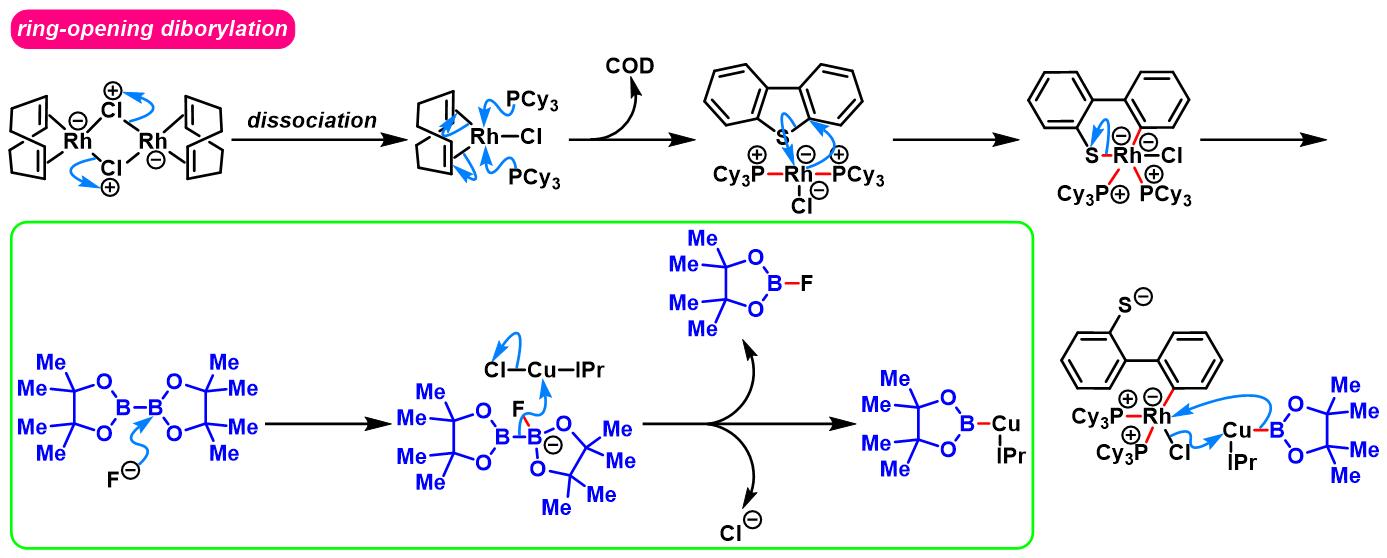
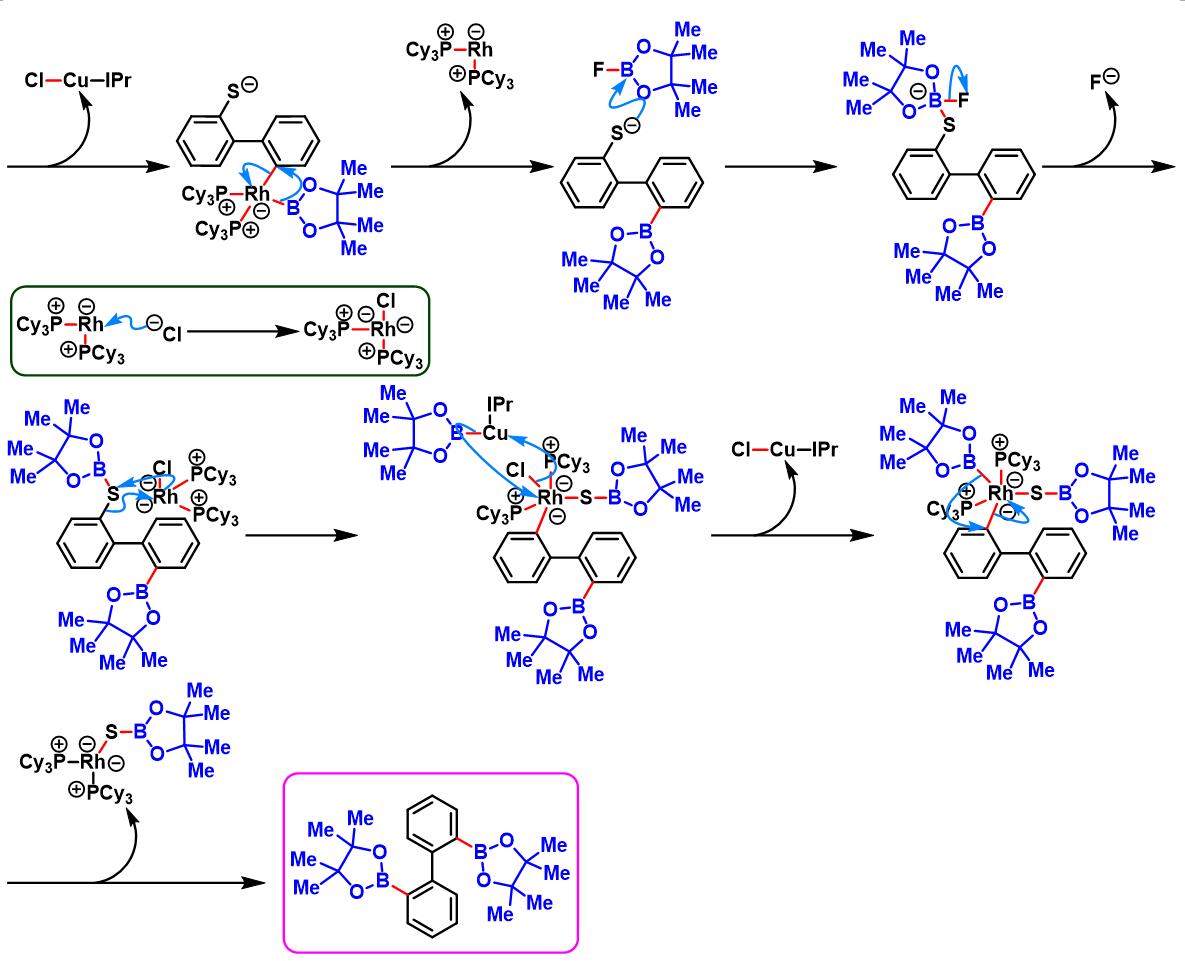
1. 二苯并噻吩参与的aromatic metamorphosis[1]-[15]
(a) 生成三亚苯
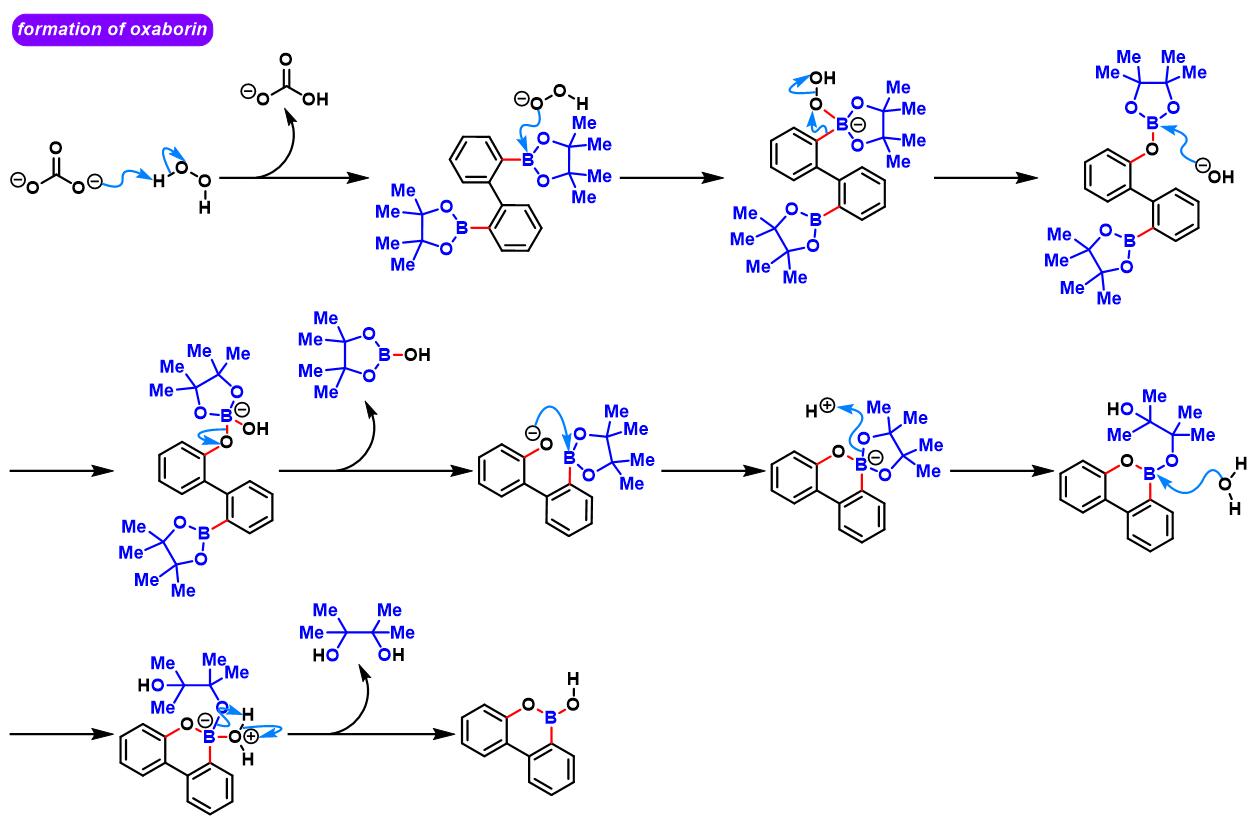
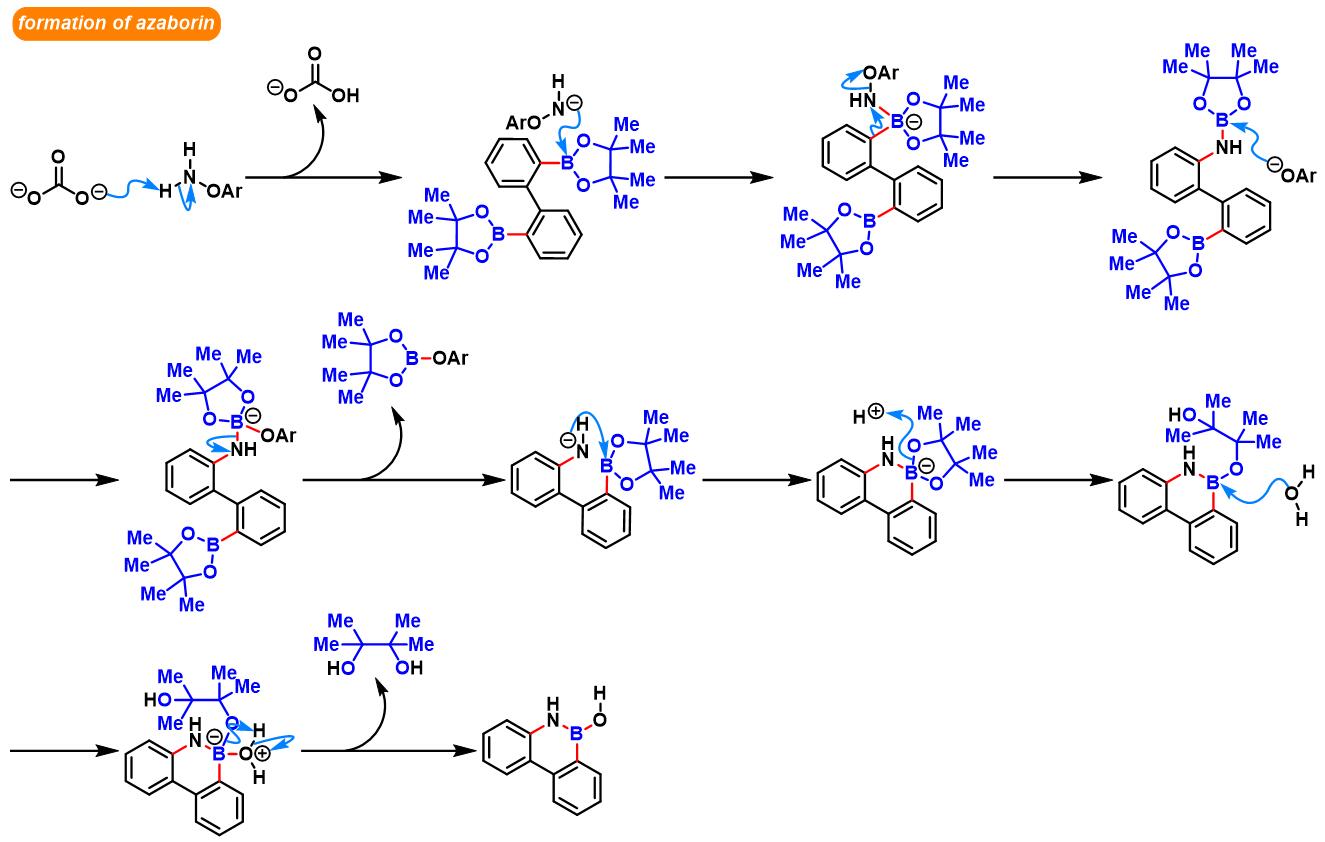
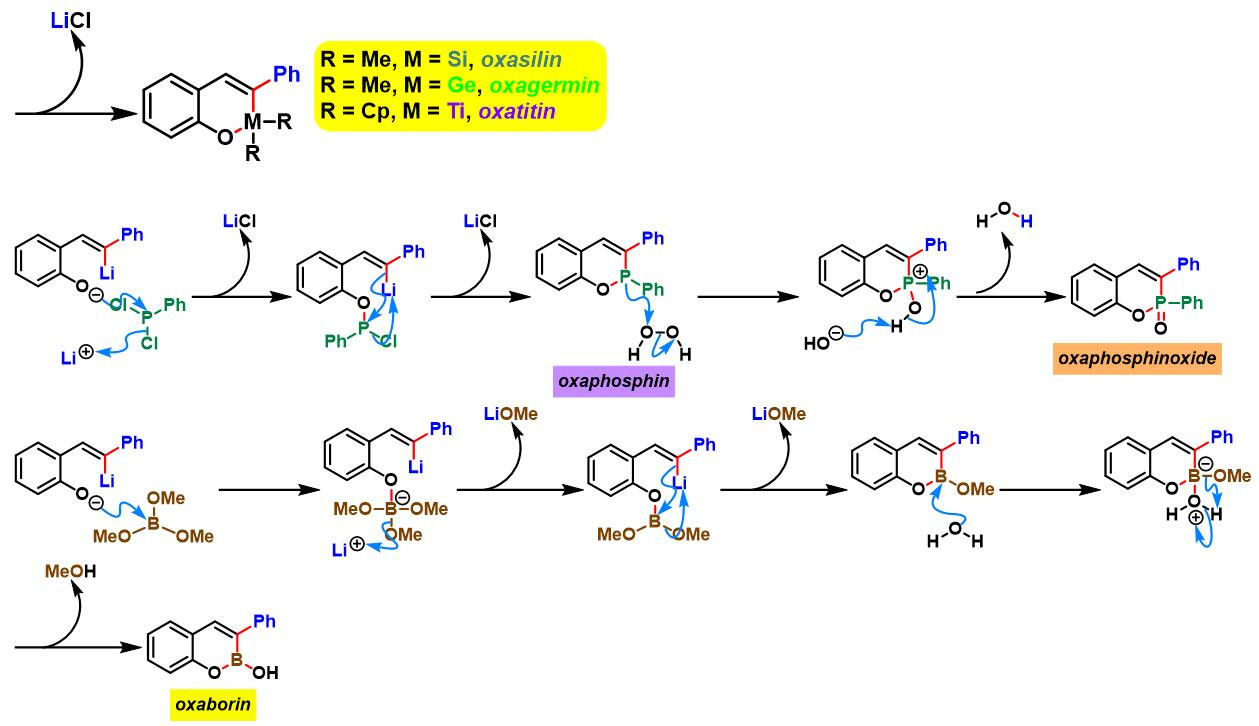
(b) 生成苯并呋喃与oxaborin及azaborin
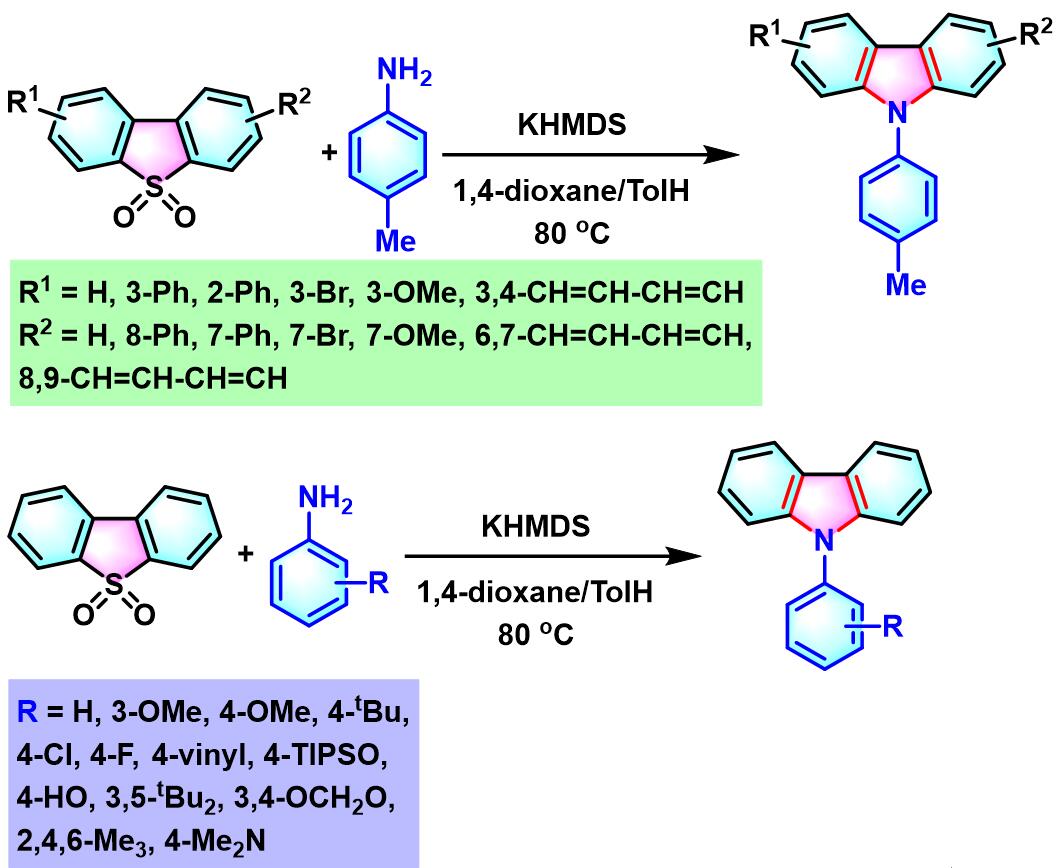
2. 二苯并噻吩二氧化物参与的aromatic metamorphosis[16]-[20]
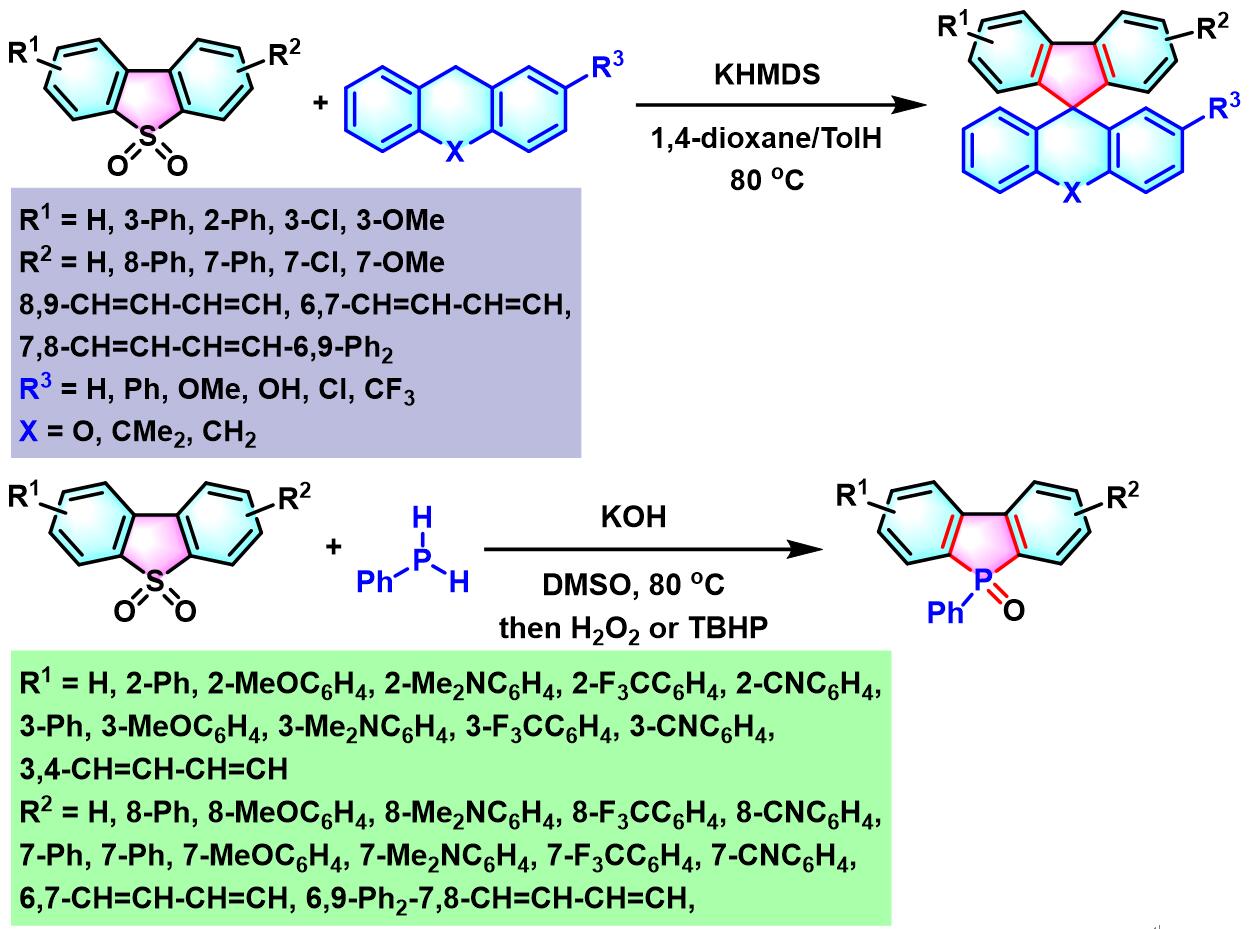
(a) 生成咔唑
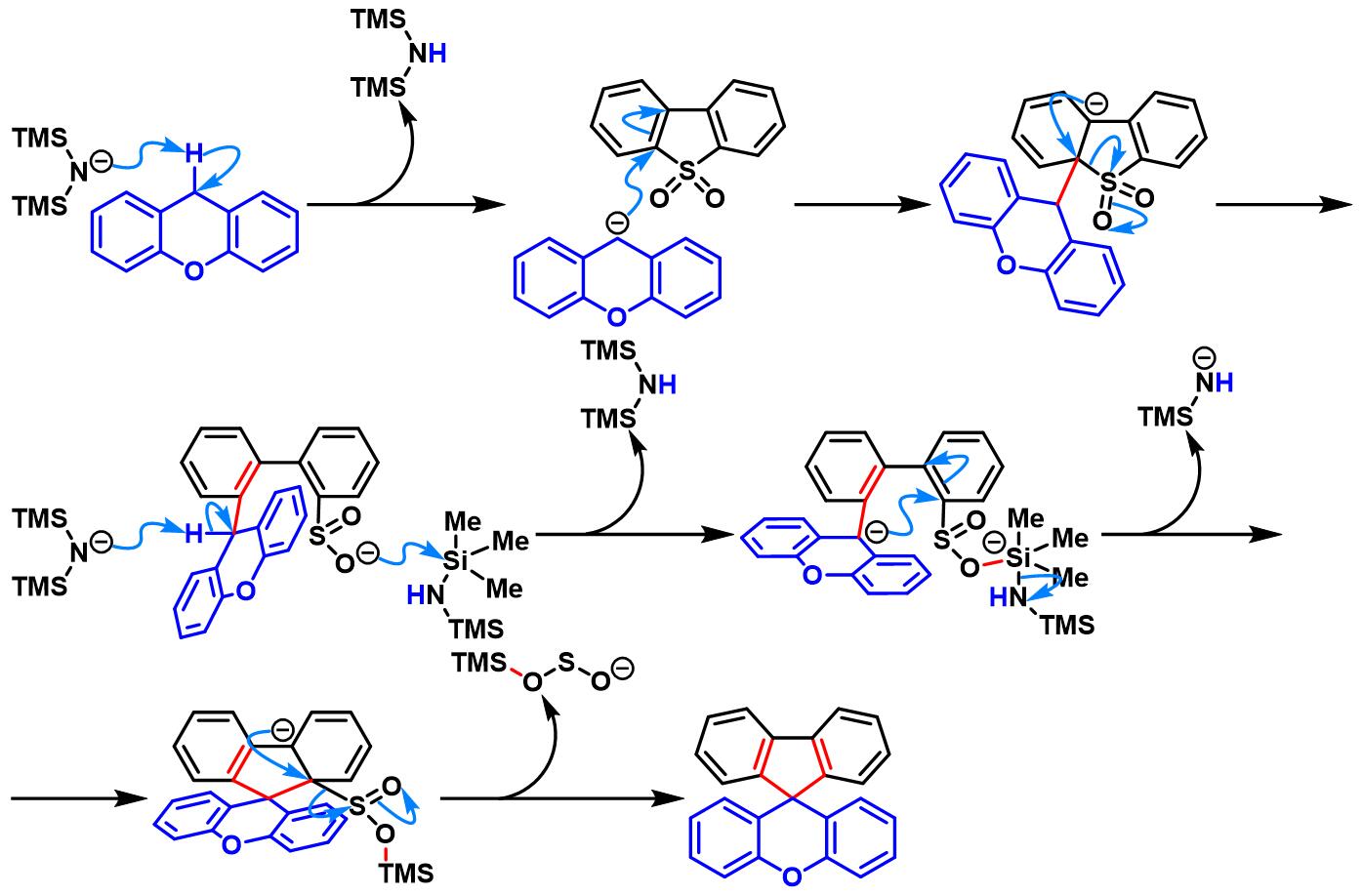
(b) 生成螺环二芳基芴 (spirocyclic diarylfluorene)
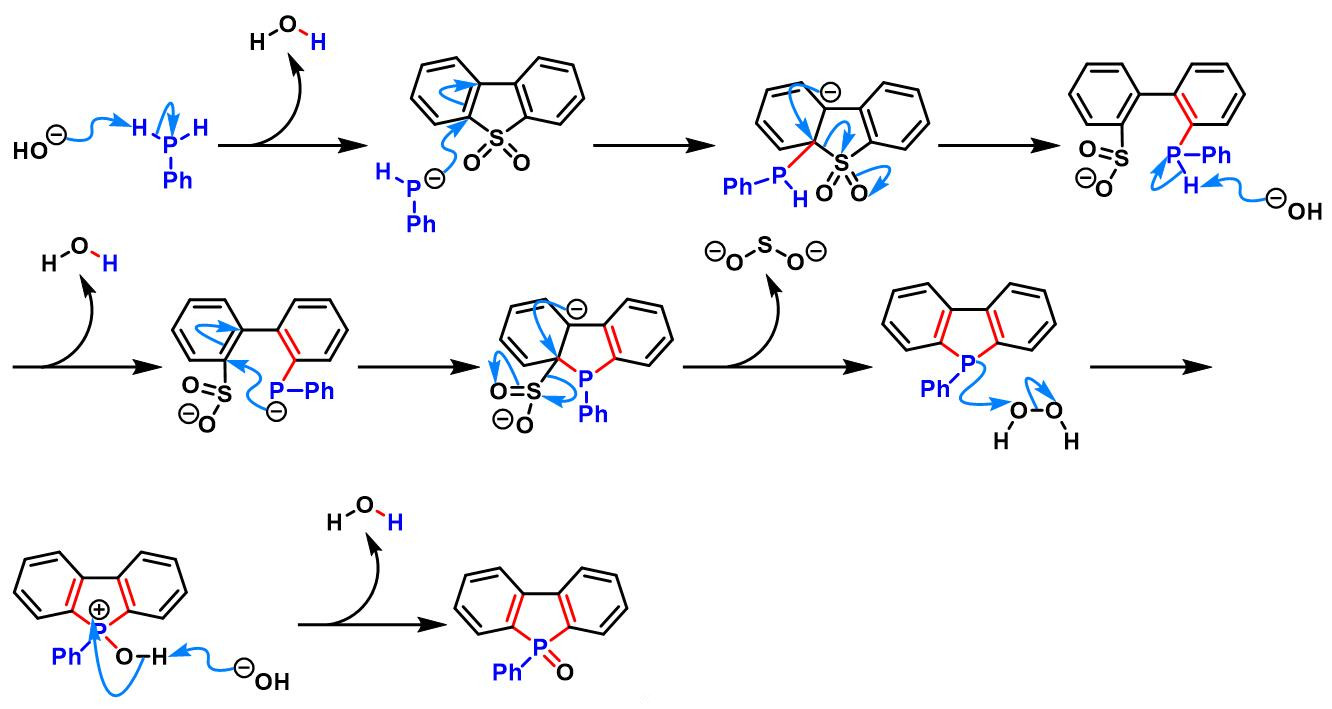
(c) 生成dibenzophosphole oxide
3. 苯并呋喃参与的aromatic metamorphosis[21]-[27]
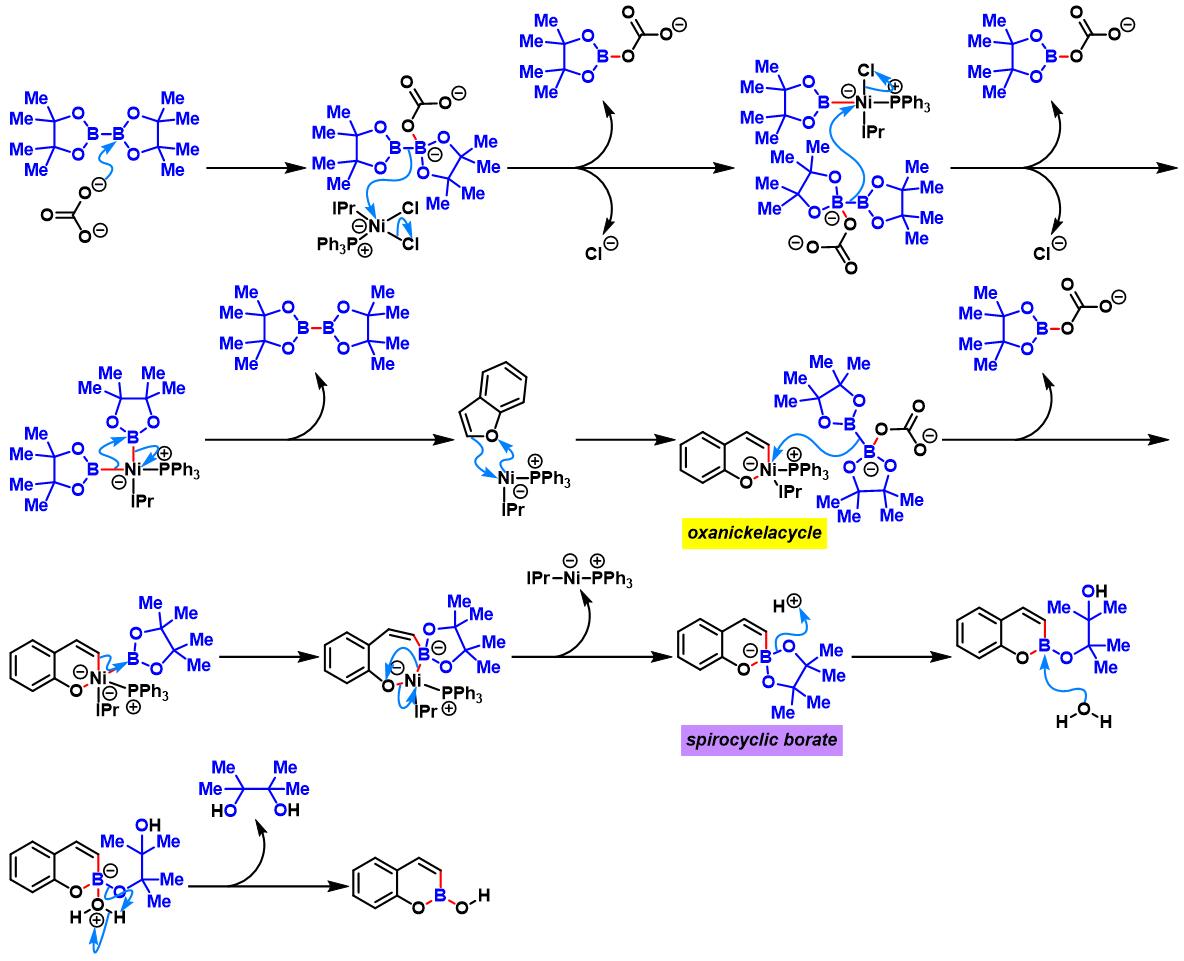
(a) 生成oxaborin
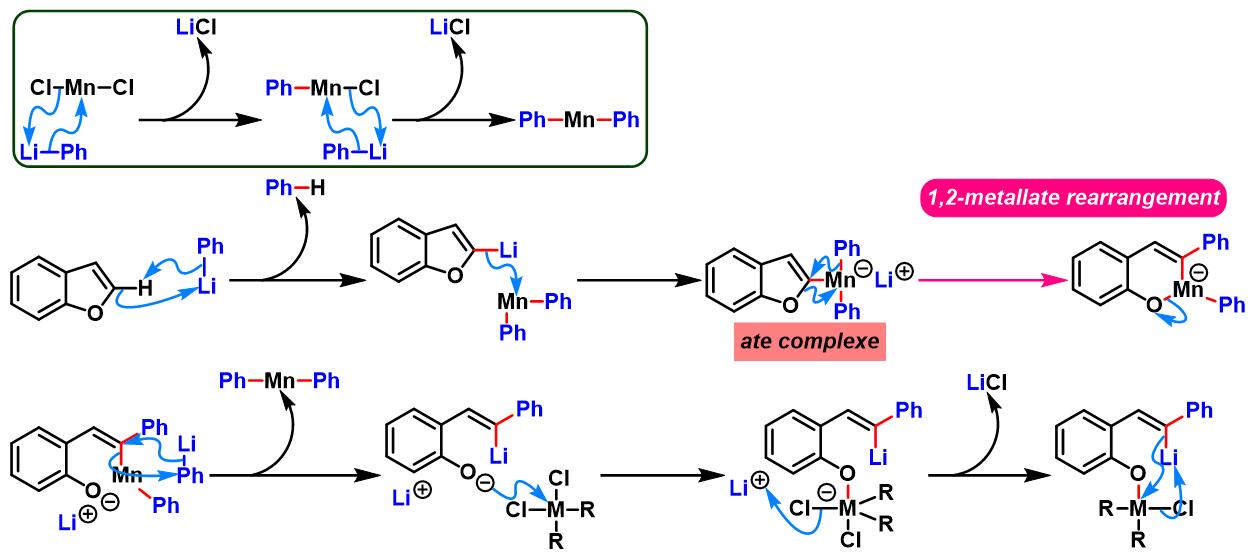
(b) 生成其它六元杂环
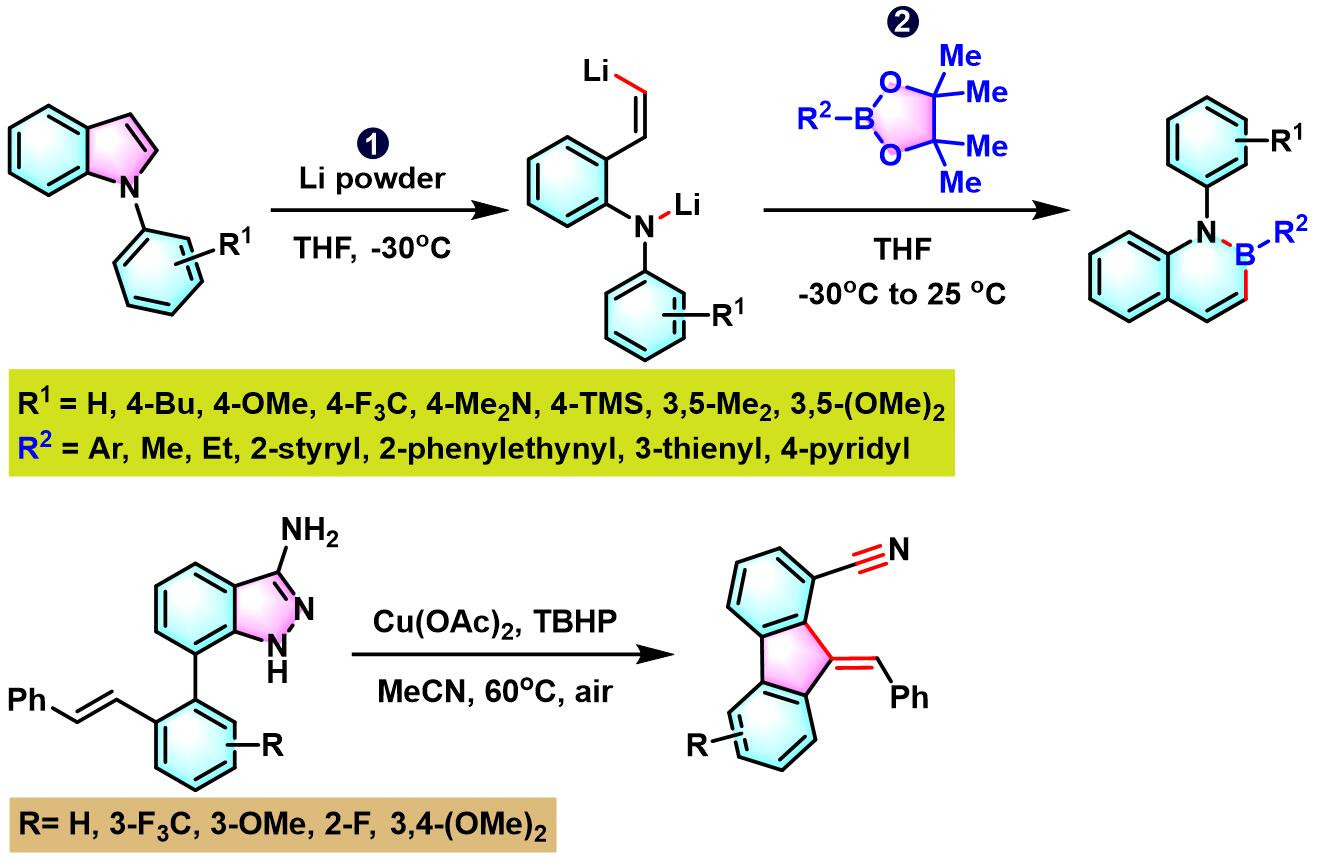
4. 二苯并呋喃参与的aromatic metamorphosis[22], [28]-[36]
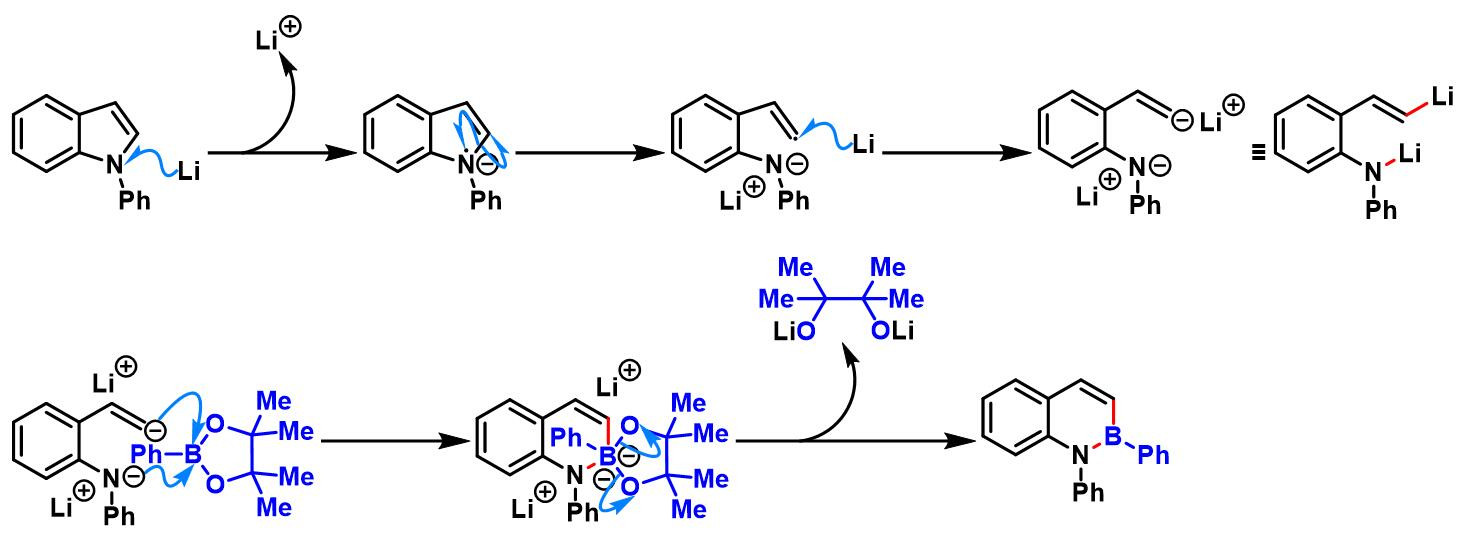
 5. 吲哚参与的aromatic metamorphosis[37]
5. 吲哚参与的aromatic metamorphosis[37]
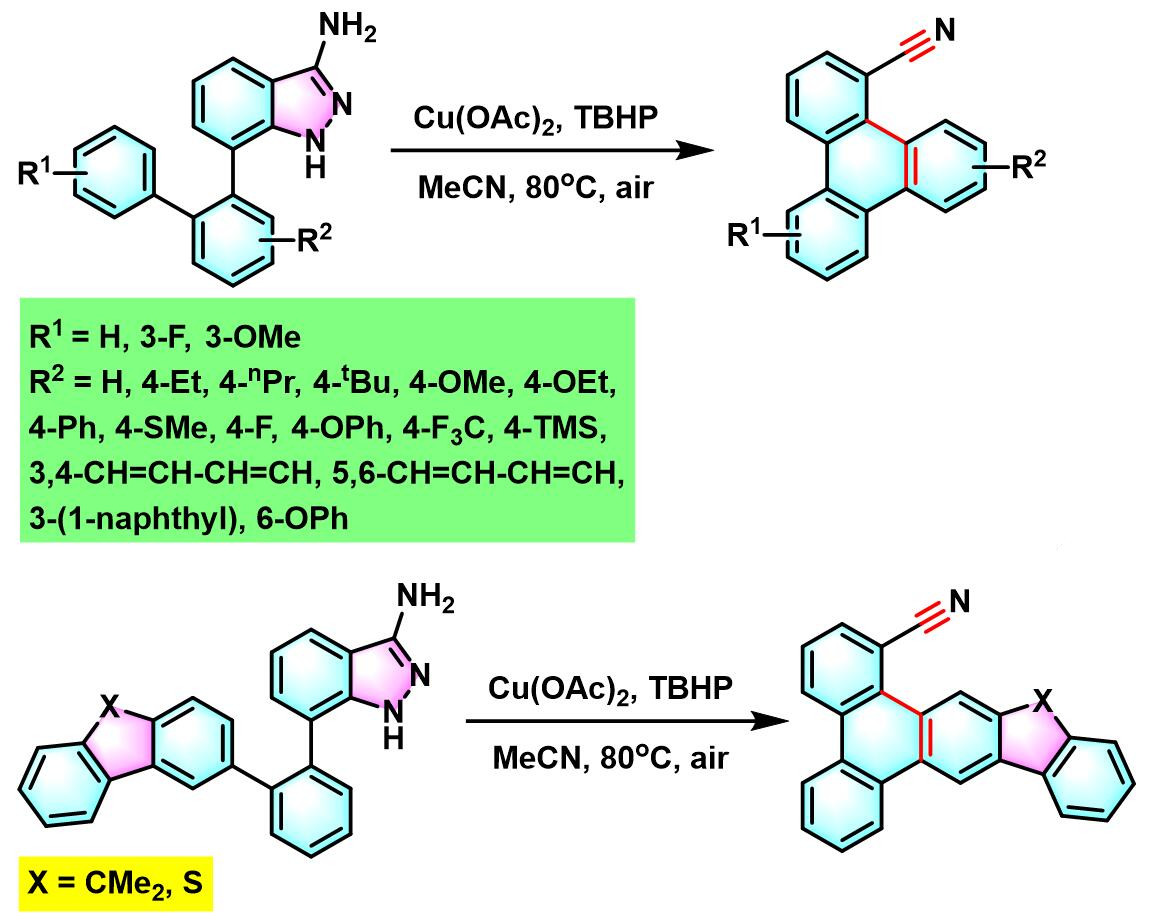
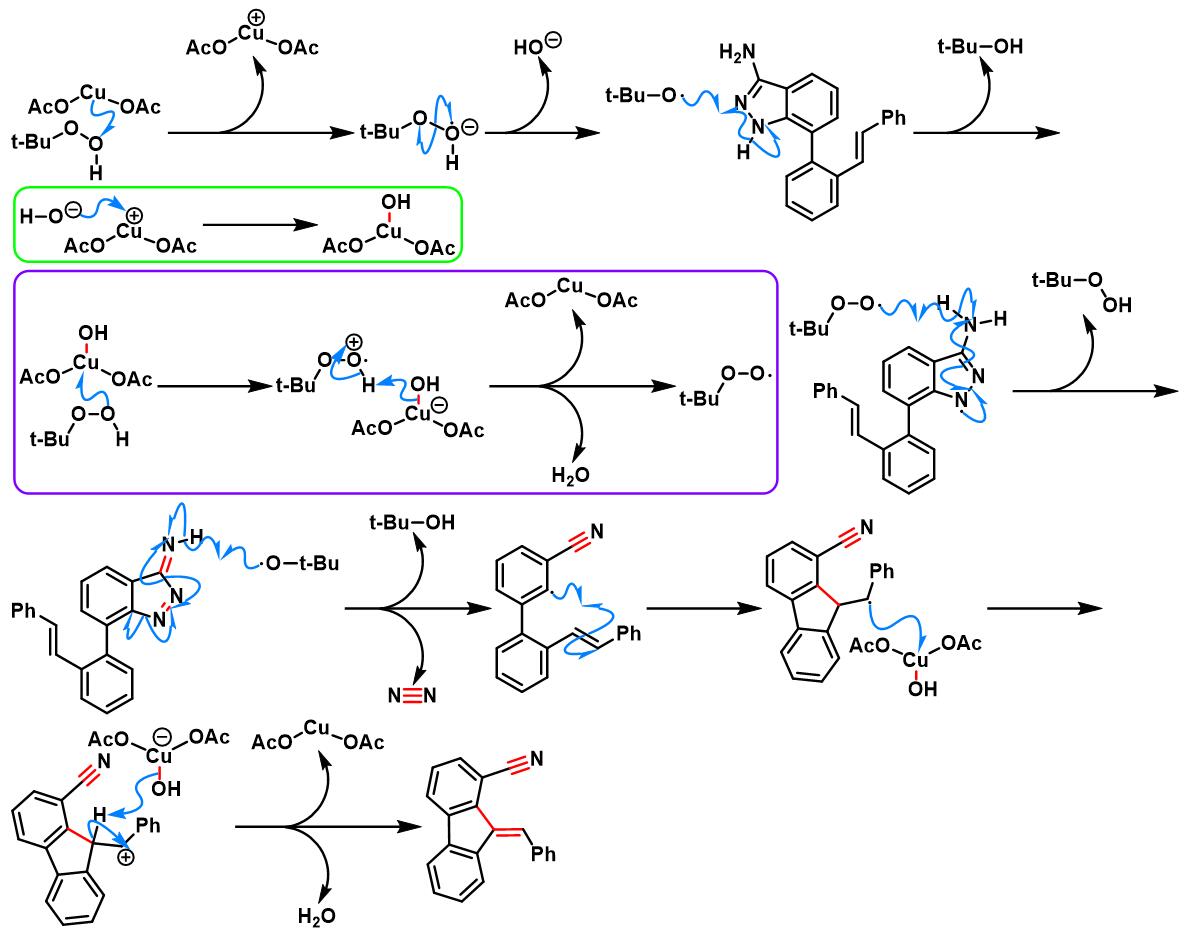
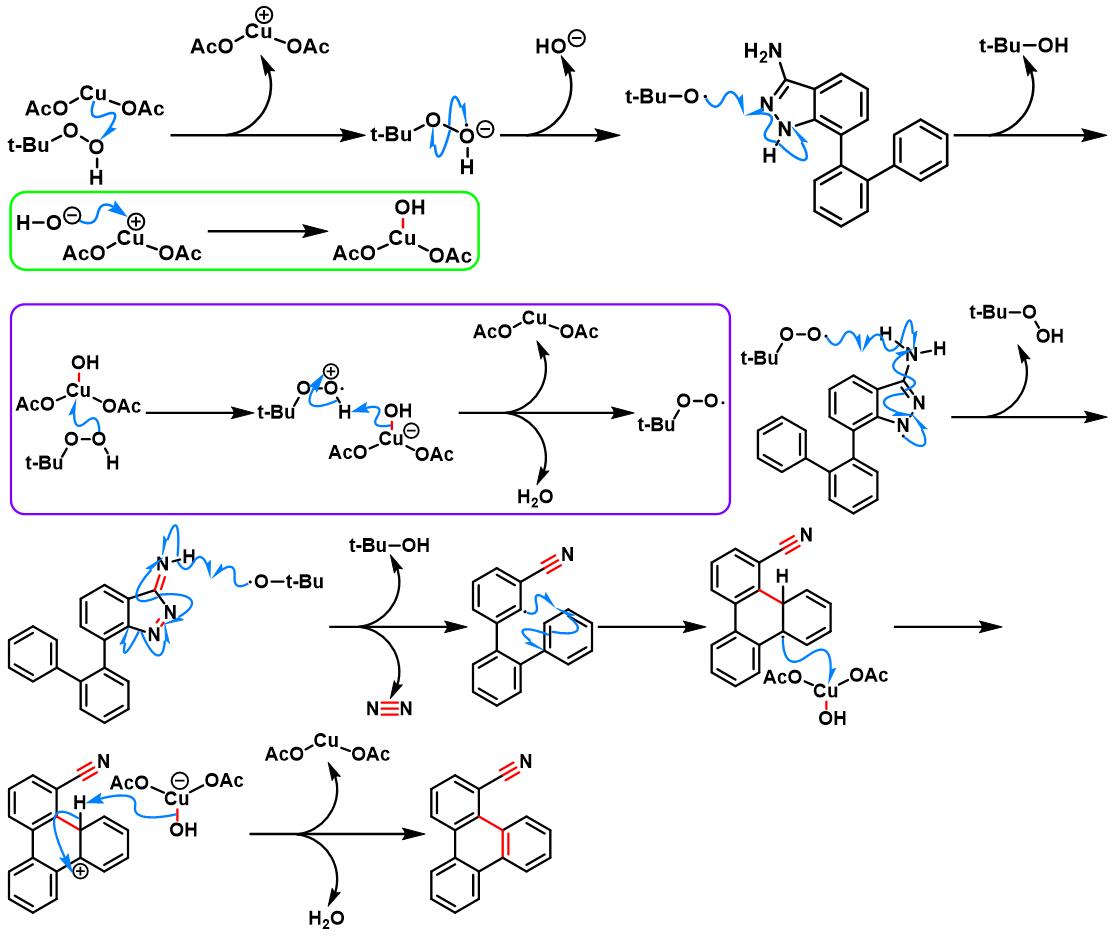
6. 氨基吲唑参与的aromatic metamorphosis[38]-[39]
(a) 生成芴
(b) 生成三亚苯
参考文献
- [1] K. K. Laali, J. Chun, T. Okazaki, S. Kumar, G. L. Borosky, C. Swartz, J. Org. Chem. 2007, 72, 8383. doi: 10.1021/jo701502y.
- [2] F. Zhu, Z. Wang, Org. Lett. 2015, 17, 1601. doi: 10.1021/acs.orglett.5b00510.
- [3] M. Gomez-Gallego, M. A. Sierra, Chem. Rev. 2011, 111, 4857. doi: 10.1021/cr100436k.
- [4] H. Minami, K. Nogi, H. Yorimitsu, Org. Lett. 2019, 21, 2518. doi: 10.1021/acs.orglett.9b00067.
- [5] D. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174. doi: 10.1021/cr0509760.
- [6] T. Satoh, M. Miura, Chem. Lett. 2007, 36, 200. doi: 10.1246/cl.2007.200.
- [7] M. Bhanuchandra, A. Baralle, S. Otsuka, K. Nogi, H. Yorimitsu, A. Osuka, Org. Lett. 2016, 18, 2966. doi: 10.1021/acs.orglett.6b01305.
- [8] Y. Uetake, T. Niwa, T. Hosoya, Org. Lett. 2016, 18, 2758. doi: 10.1021/acs.orglett.6b01250.
- [9] T. Niwa, H. Ochiai, Y. Watanabe, T. Hosoya, J. Am. Chem. Soc. 2015, 137, 14313. doi: 10.1021/jacs.5b10119.
- [10] C. Zarate, R. Martin, J. Am. Chem. Soc. 2014, 136, 2236. doi: 10.1021/ja412107b.
- [11] A. N. Desnoyer, J. A. Love, Chem. Soc. Rev. 2017, 46, 197. doi: 10.1039/C6CS00150E.
- [12] D. A. Vicic, W. D. Jones, J. Am. Chem. Soc. 1999, 121, 7606. doi: 10.1021/ja9905997.
- [13] J. C. Vantourout, H. N. Miras, A. Isidro-Llobet, S. Sproules, A. J. B. Watson,. Am. Chem. Soc. 2017, 139, 13, 4769. doi: 10.1021/jacs.6b12800.
- [14] B. Q. Xiong, Q. Cheng, C. H. Hu, P. L. Zhang, Y. Liu, K. W. Tang, Chemistry Select 2017, 2, 6891. doi: 10.1002/slct.201700716
- [15] C. Zhu, G. Li, D. H. Ess, J. R. Falck, L. Kürti, J. Am. Chem. Soc. 2012, 134, 18253. doi: 10.1021/ja309637r.
- [16] S. Oae, N. Furukawa, Bull. Chem. Soc. Jpn. 1966, 39, 2260. doi: 10.1246/bcsj.39.2260.
- [17] T. G. Squires, C. G. Venier, B. A. Hodgson, L. W. Chang, J. Org. Chem. 1981, 46, 2373. doi: 10.1021/jo00324a031.
- [18] T. Aida, T. G. Squires, C. G. Venier, Tetrahedron Lett. 1983, 24, 3543. doi: 10.1016/S0040-4039(00)88163-0.
- [19] P. J. Bruna, F. Grein, J. Phys. Chem. A. 2012, 116, 10229. doi: 10.1021/jp307322j.
- [20] Denney, D. B. Goodyear, W. F. B. Goldstein, J. Am. Chem. Soc. 1960, 82, 1393. doi: 10.1021/ja01491a027.
- [21] H. Saito, H. Yorimitsu, Chem. Lett. 2019, 48, 1019. doi: 10.1246/cl.190393.
- [22] M. Tobisu, N. Chatani, Acc. Chem. Res. 2015, 48, 1717. doi: 10.1021/acs.accounts.5b00051.
- [23] T. Nguyen, E. Negishi, Tetrahedron Lett. 1991, 32, 5903. doi: 10.1016/S0040-4039(00)79422-6.
- [24] H. Kakiya, R. Inoue, H. Shinokubo, K. Oshima, Tetrahedron 2000, 56, 2131. doi: 10.1016/S0040-4020(99)01095-9.
- [25] G. Cahiez, C. Duplais, J. Buendia, Chem. Rev. 2009, 109, 3, 1434. doi: 10.1021/cr800341a.
- [26] F. Mongin, A, Harrison-Marchand, Chem. Rev. 2013, 113, 10, 7563. doi: 10.1021/cr3002966.
- [27] M. Tobisu, T. Morioka, A. Ohtsuki, N. Chatani, Chem. Sci. 2015, 6, 3410. doi: 10.1039/C5SC00305A.
- [28] J. Cornella, R. Martin, Org. Lett. 2013, 15, 6298. doi: 10.1021/ol4031815.
- [29] J. Cornella, E. Gomez-Bengoa, R. Martin, J. Am. Chem.Soc. 2013, 135, 1997. 10.1021/ja311940s
- [30] J. Cornella, C. Zarate, R. Martin, Chem. Soc. Rev. 2014, 43, 8081. doi: 10.1039/C4CS00206G.
- [31] X. Liu, C. Hsiao, I. Kalvet, M. Leiendecker, L. Guo, F. Schoenebeck, M. Rueping, Angew. Chem. Int. Ed. 2016, 55, 6093. doi: 10.1002/anie.201510497.
- [32] R. M. Letcher, K. Wong, J. Chem. Soc. Perkin 1, 1977, 178. doi: 10.1039/P19770000178.
- [33] R. Srinivasan, V. Y. Merritt, J. N. C. Hsu, P. H. G. op het Veld, W. H. Laarhoven, J. Org. Chem.1978, 43, 980. doi: 10.1021/jo00399a039.
- [34] I. Schnapperelle, T. Bach, Chem. Eur. J. 2014, 20, 9725. doi: 10.1002/chem.201402765.
- [35] J. Grimshaw, A. P. de Silva, Chem. Soc. Rev. 1981, 10, 181. doi: 10.1039/CS9811000181.
- [36] M. P. Cava, M. J. Mitchell, S. C. Havlicek, A. Lindert, R. J. Spangler, J. Org. Chem. 1970, 35, 175. doi: 10.1021/jo00826a038.
- [37] M. Yus, F. Foubelo, J. V. Ferrández, Eur. J. Org. Chem. 2001, 2809. doi: 10.1002/1099-0690(200108)2001:15<2809::AID-EJOC2809>3.0.CO;2-T.
- [38] G. Rothenberg, L. Feldberg, H. Wiener, Y. Sasson, J. Chem. Soc. Perkin Trans. 2 1998, 2, 2429. doi: 10.1039/A805324C.
- [39] Y. Zhou, L. Lin,Y. Wang, J. Zhu, Q. Song, Org. Lett. 2019, 21, 7630. doi: 10.1021/acs.orglett.9b02933.
反应实例
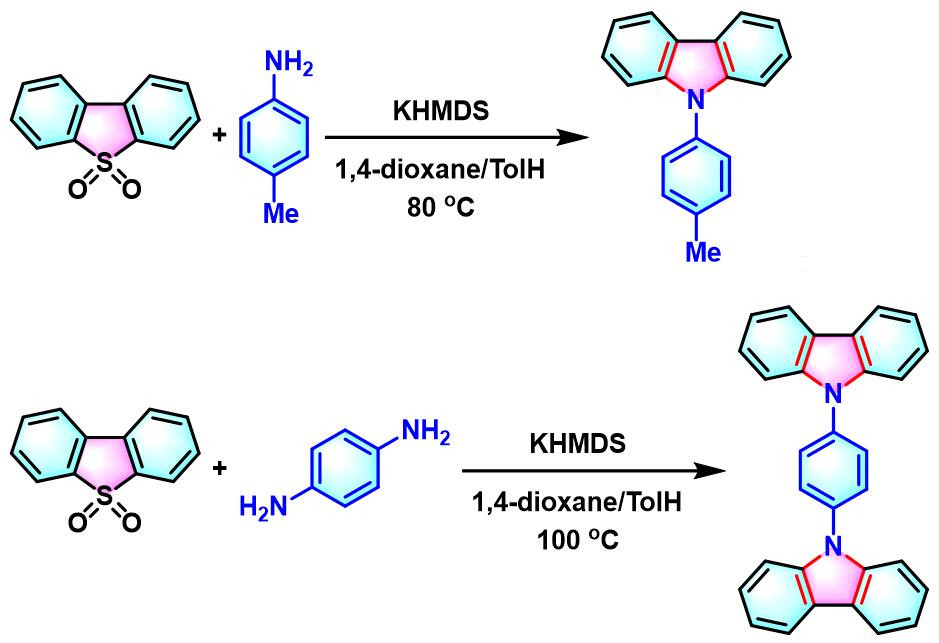
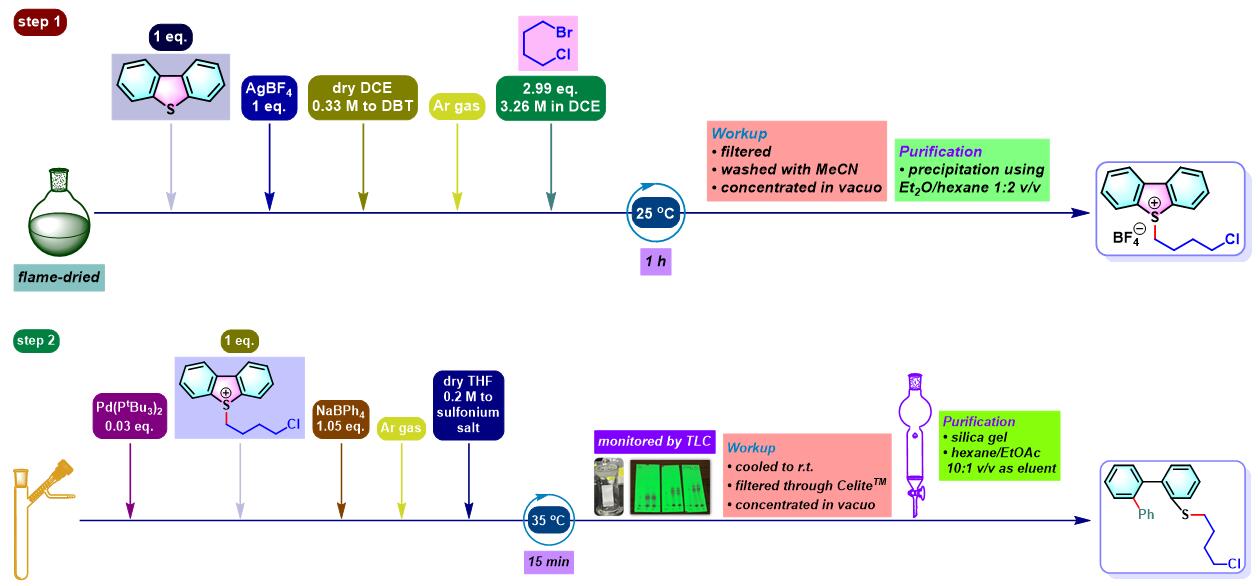
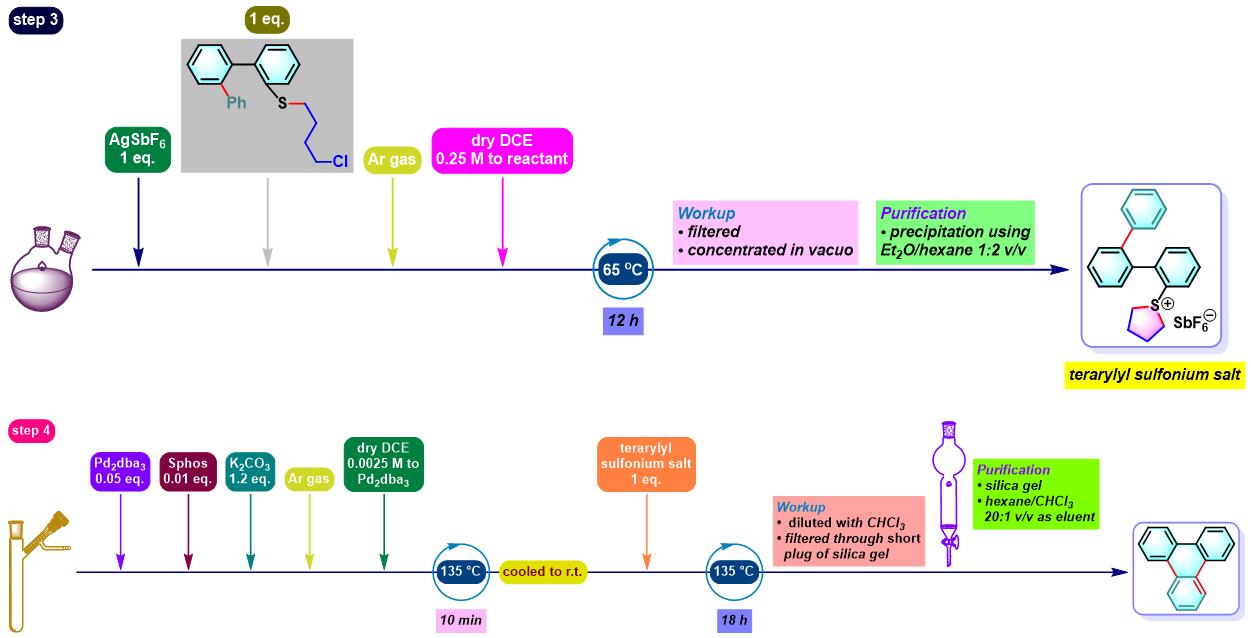
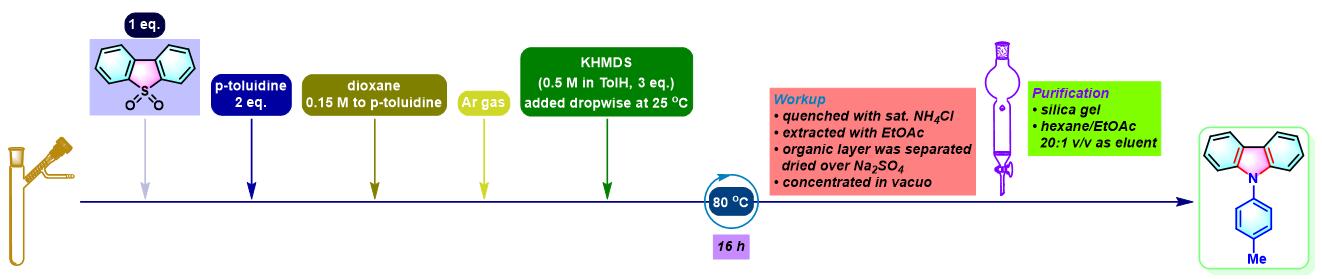
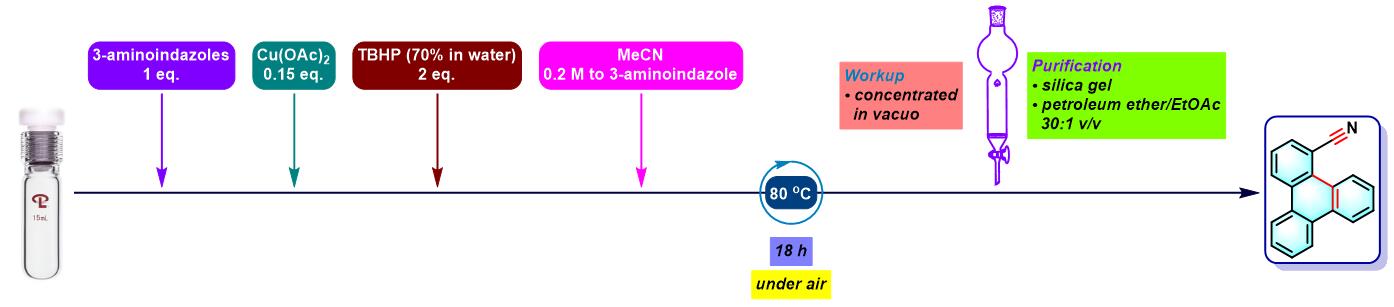
咔唑的合成[1]
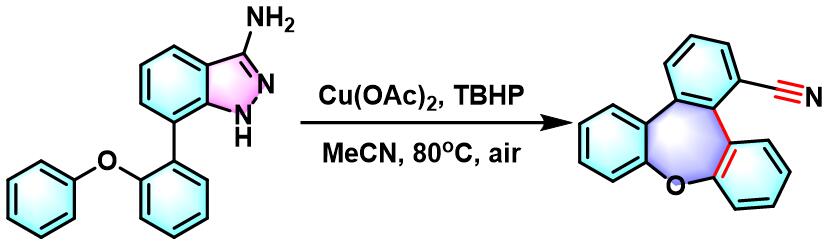
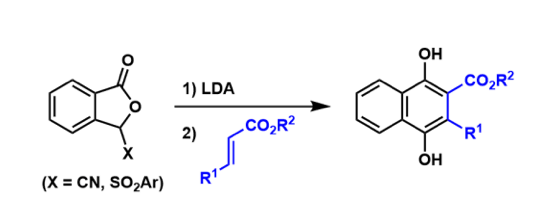
tribenzo[b,d,f ]oxepine-1-carbonitrile的合成[2]
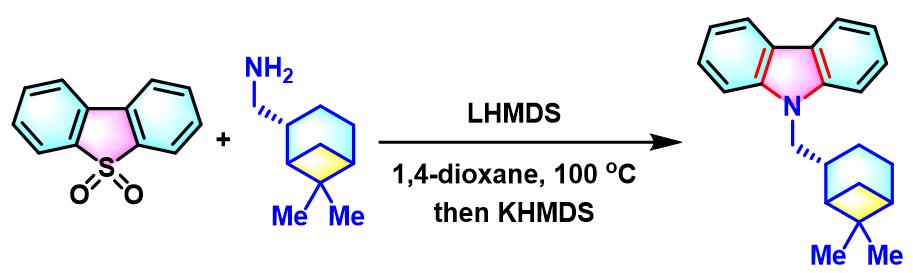
N-烷基咔唑的合成[3]
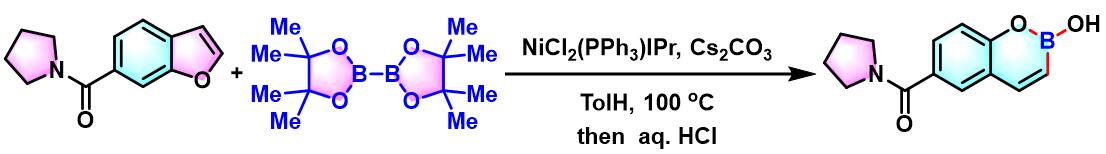
oxaborin的合成[4]
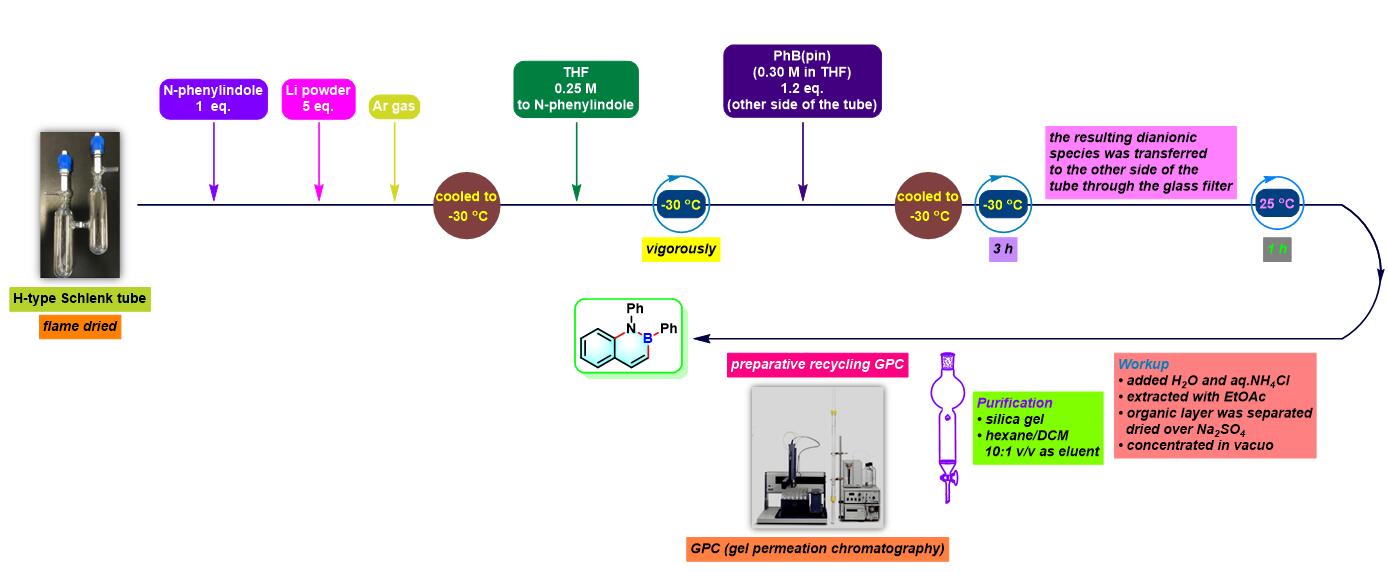
azaborin的合成[5]
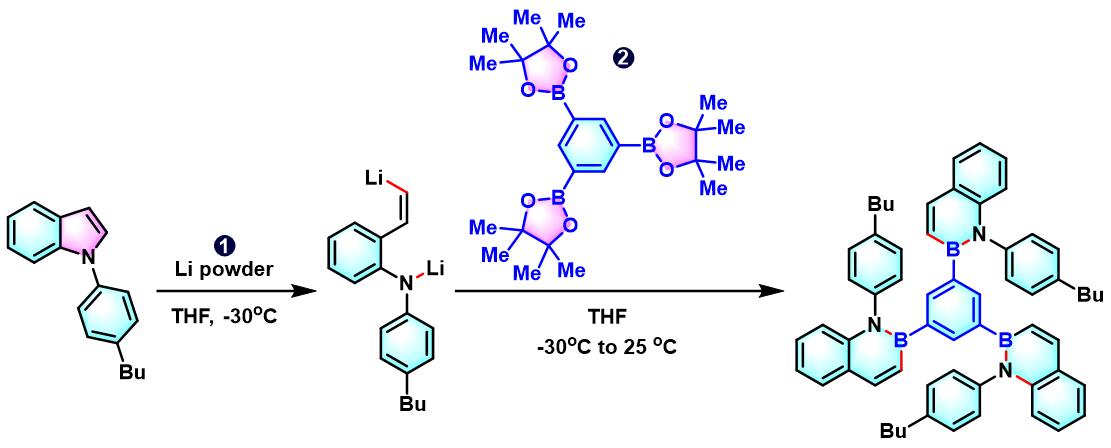
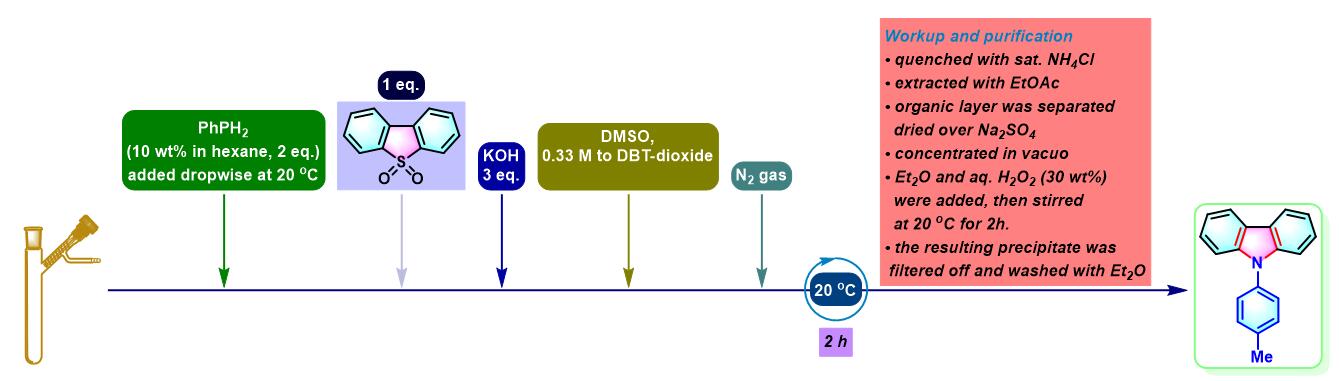
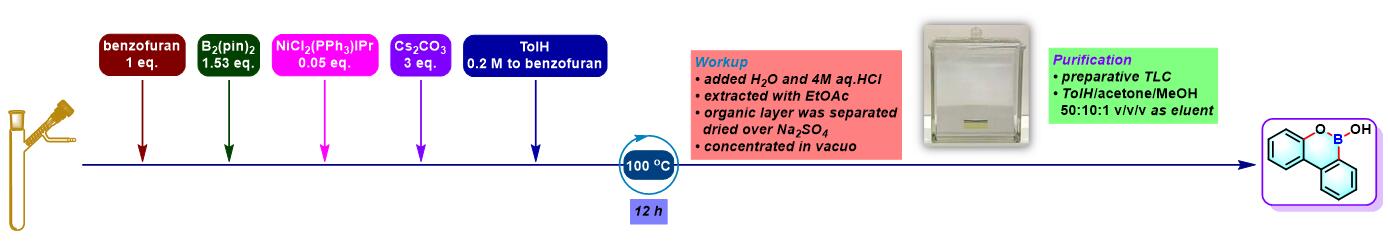
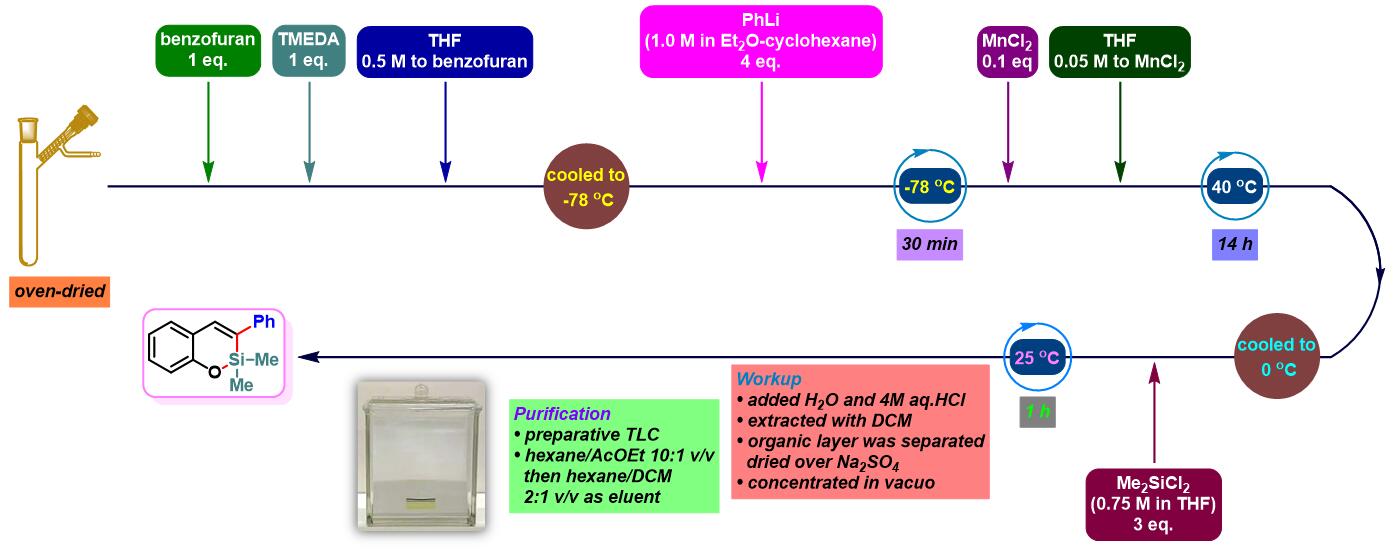
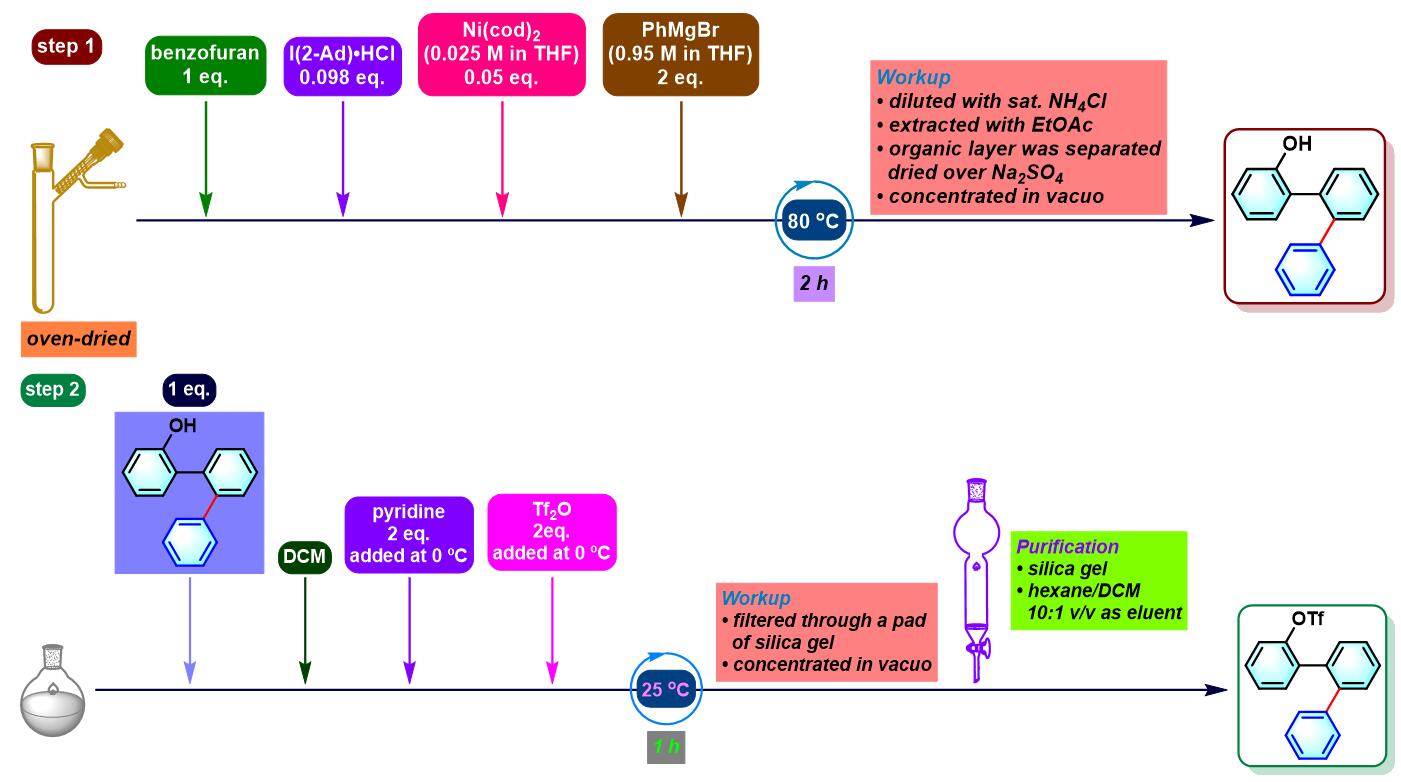
实验步骤
1. 二苯并噻吩参与的aromatic metamorphosis
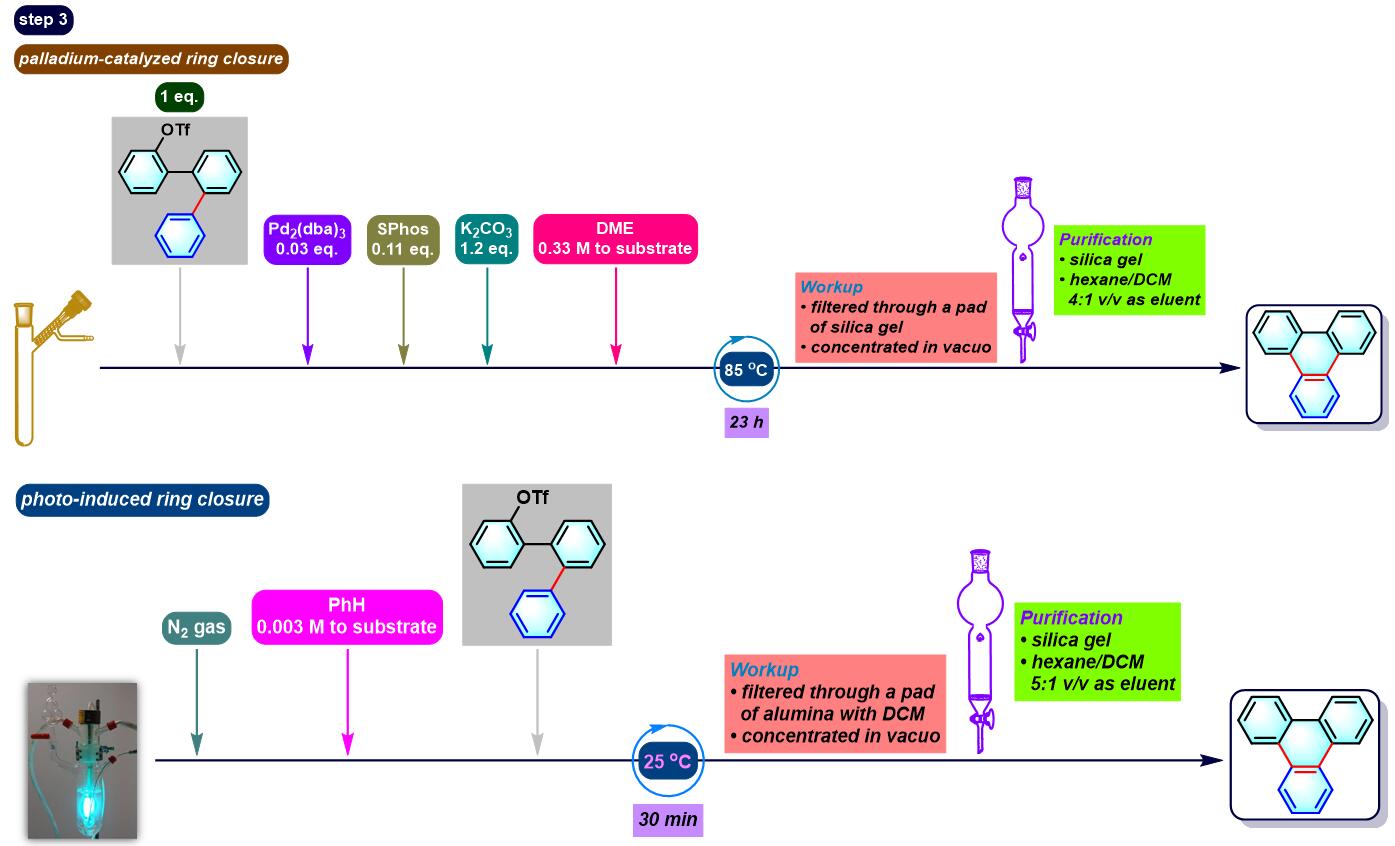
(a) 生成三亚苯
(b) 生成苯并呋喃与oxaborin及azaborin
2. 二苯并噻吩二氧化物参与的aromatic metamorphosis
(a) 生成咔唑
(b) 生成螺环二芳基芴
(c) 生成dibenzophosphole oxide
3. 苯并呋喃参与的aromatic metamorphosis
(a) 生成oxaborin
(b) 生成oxasilin
4. 二苯并呋喃参与的aromatic metamorphosis
5. 吲哚参与的aromatic metamorphosis
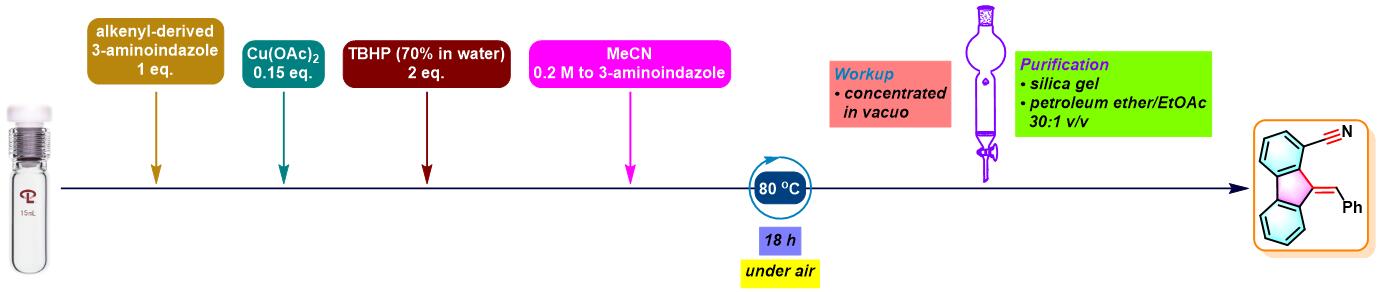
6. 氨基吲唑参与的aromatic metamorphosis
(a) 生成芴
(b) 生成三亚苯
参考文献
- [1] M. Bhanuchandra, K. Murakami, D. Vasu, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2015, 54, 10234. doi: 10.1002/anie.201503671.
- [2] Y. Zhou, L. Lin,Y. Wang, J. Zhu, Q. Song, Org. Lett. 2019, 21, 7630. doi: 10.1021/acs.orglett.9b02933.
- [3] A. Kaga, K. Nogi, H. Yorimitsu, Chem. Eur. J. 2019, 25, 14780. doi: 10.1002/chem.201903916.
- [4] H. Saito, S. Otsuka, K. Nogi, H. Yorimitsu, J. Am. Chem. Soc. 2016, 138, 15315. doi: 10.1021/jacs.6b10255.
- [5] S. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett. 2019, 21, 3855. doi: 10.1021/acs.orglett.9b01353.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!






















No comments yet.