本文作者:asymmboy
Chem-station小编继续为学习有机化学的各位同行带来Fukuyama-Yokoshima研究组反应机理问题的详细解答的第十六期。
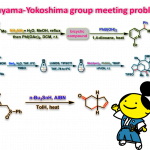
问题 1
基本文献
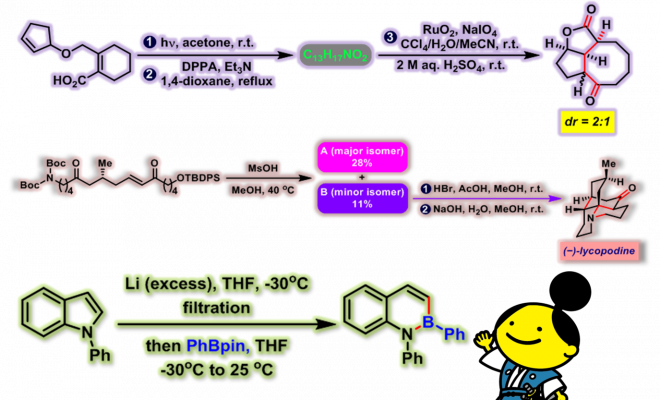
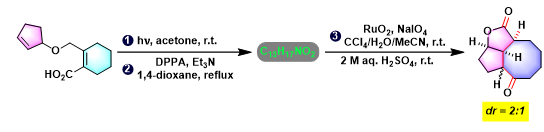
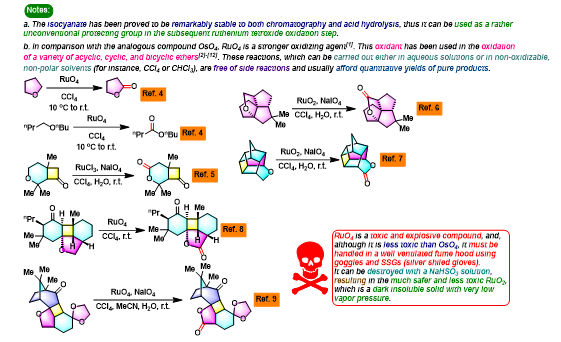
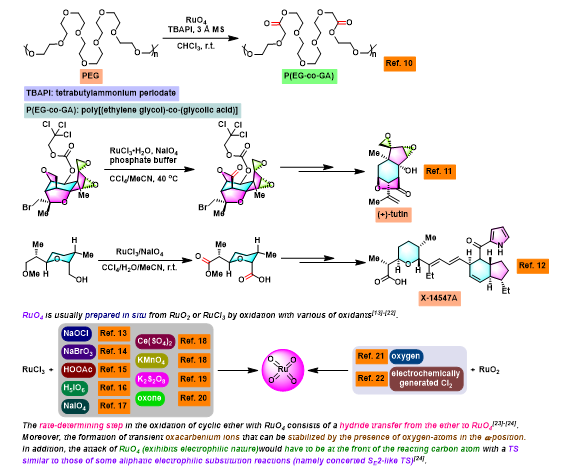
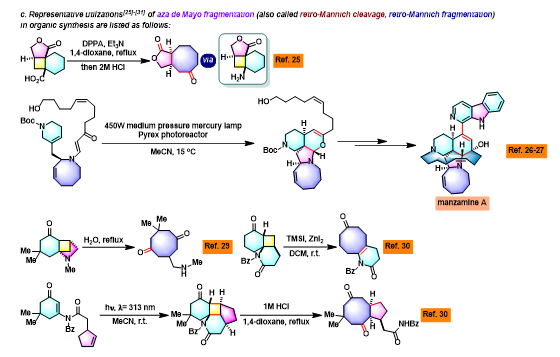
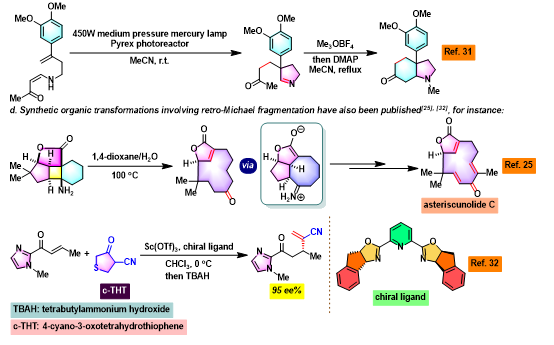
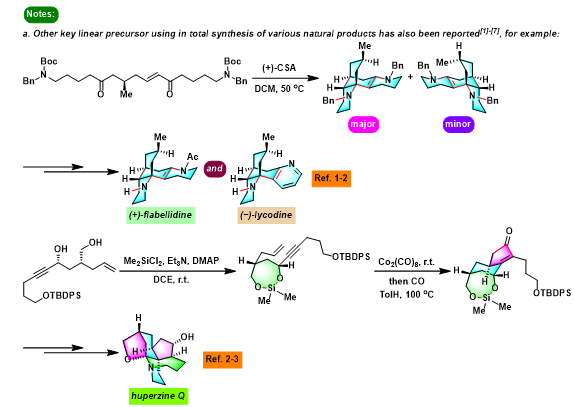
[1] K. I. Booker-Milburn, J. K. Cowell, Tetrahedron Lett. 1996, 37, 2177. doi: 10.1016/0040-4039(96)00224-9.相关链接: 四氧化钌
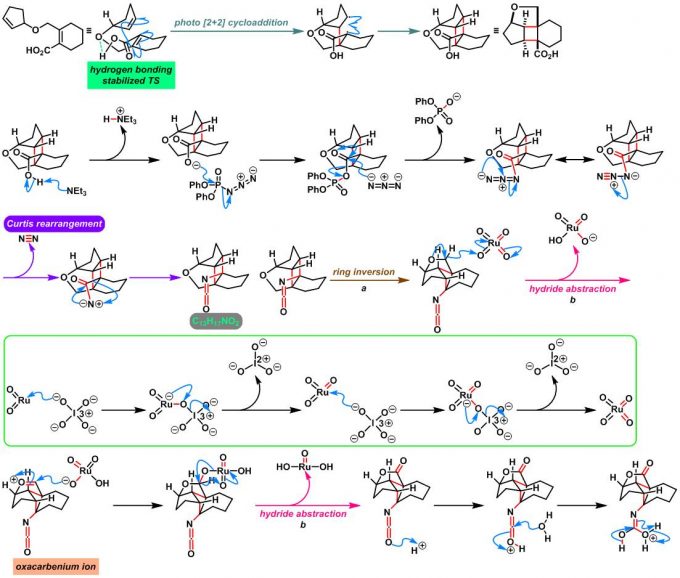
反应机理
参考文献
[1] C. Djerassi, R. R. Engle, J. Am. Chem. Soc. 1953, 75, 3838. doi: 10.1021/ja01111a507. [2] D. G. Lee, M. Van den Engh, Can. J. Chem. 1972, 50, 3129. doi: 10.1139/v72-501. [3] A. Tenaglia, E. Terranova, B. Waegell, J. Chem. Soc. Chem. Commun. 1990, 19, 1344. doi: 10.1039/C39900001344. [4] L. M. Berkowitz, P. N. Rylander, J. A m. Chem. Soc. 1958, 80, 6682. doi: 10.1021/ja01557a053. [5] D. Johnston, K. N. Slessor, A. C. Oehlschlager, J. Org. Chem. 1985, 50, 114. doi: 10.1021/jo00201a022. [6] B. Smith, III, B. A. Wexter, C. Y. Tu, J. P. Konepelski, J. Am. Chem. Soc. 1985, 107, 1308. doi: 10.1021/ja00291a034. [7] W. G. Dauben, A. F. Cunningham Jr, J. Org. Chem. 1983, 48, 2842. doi: 10.1021/jo00165a012. [8] Kojima, Y. Inouye, H. Kakisawa, Bull. Chem. Soc. Jpn. 1985, 58, 1738. doi: 10.1246/bcsj.58.1738. [9] W. F. Berkowitz, A. S. Amarasekara, J. J. Perumattam, J. Org. Chem. 1987, 52, 1119. doi: 10.1021/jo00382a026. [10] D. Liu, C. W. Bielawski. Macromol. Rapid Commun. 2016, 37, 1587. doi: 10.1002/marc.201600336. [11] K. Wakamatsu, H. Kigoshi, K. Niiyama, H. Niwa, K. Yamada, Tetrahedron 1986, 42, 5551. doi: 10.1016/S0040-4020(01)88159-X. [12] R. A. Whitney, Can. J. Chem. 1986, 64, 803. doi: 10.1139/v86-132. [13] H. Gopal, A. J. Gordon, Tetrahedron Lett.1971, 12, 2941. doi: 10.1016/S0040-4039(01)97031-5. [14] Y. Yamamoto, H. Suzuki, Y. Moro-oka, Tetrahedron Lett. 1985, 26, 2107. doi: 10.1016/S0040-4039(00)94791-9. [15] C. Guizard, H. Cheradame, Y. Brunel, C. G. Beguin, J. Fluorine Chem. 1979, 13, 175. doi: 10.1016/S0022-1139(00)81086-6. [16] C. M. Rodríguez, J. M. Ode, J. M. Palazón, V. S. Martín, Tetrahedron1992, 48, 3571. doi: 10.1016/S0040-4020(01)88494-5. [17] P. H. J. Carlsen, T. Katsuki, V. S. Martin, K. B. Sharpless, J. Org. Chem. 1981, 46, 3936. doi: 10.1021/jo00332a045. [18] S. Giddings, A. Mills, J. Org. Chem. 1988, 53, 1103. doi: 10.1021/jo00240a037. [19] L. A. Paquette, J. Dressel, P. D. Pansegran, Tetrahedron Lett.1987, 28, 4965. doi: 10.1016/S0040-4039(00)96671-1. [20] S. Torii, T. Inokuchi, T. Sugiura, J. Org. Chem. 1986, 51, 155. doi: 10.1021/jo00352a006. [26] M. Matsumoto, N. Watanabe, J. Org. Chem. 1984, 49, 3435. doi: 10.1021/jo00192a054. [22] B. Plietker, Org. Lett. 2004, 6, 289. doi: 10.1021/ol0362663. [23] M. Pedrón, L. Legnani, M.-A. Chiacchio, P. Caramella, T. Tejero, P. Merino. Beilstein J. Org. Chem. 2019, 15, 1552. doi: 10.3762/bjoc.15.158. [24] J. M. Bakke, A. E. Frøhaug, J. Phys. Org. Chem.1996, 9, 310. doi: 10.1002/(SICI)1099-1395(199606)9:6<310::AID-POC790>3.0.CO;2-E. [25] K. I. Booker-Milburn, J. K. Cowell, L. J. Harris, Tetrahedron. 1997, 53, 12319. doi: 10.1016/S0040-4020(97)00769-2. [26] T. Bach, J. P. Hehn, Angew. Chem. Int. Ed. 2011, 50, 1000. doi: 10.1002/anie.201002845. [27] J. D. Winkler, J. M. Axten, J. Am. Chem. Soc. 1998, 120, 6425. doi: 10.1021/ja981303k. [28] J. D. Winkler, C. Mazur Bowen, F. Liotta, Chem. Rev. 1995, 95, 6, 2003. doi: 10.1021/cr00038a010. [29] Y. Tamura, Y. Kita, H. Ishibashi, M. Ikeda, Tetrahedron Lett. 1972, 13, 1977. doi: 10.1016/S0040-4039(01)84766-3. [30] A. Amougay, J.-P. Pete, O. Piva, Tetrahedron Lett. 1992, 33, 7347. doi: 10.1016/S0040-4039(00)60184-3. [31] J. D. Winkler, C. L. Muller, R. D. Scott, J. Am. Chem. Soc. 1988, 110, 4831. doi: 10.1021/ja00222a053. [32] N. Duchemin, M. Cattoen, O. Gayraud, S. Anselmi, B. Siddiq, R. Buccafusca, M. Daumas, V. Ferey, M. Smietana, S. Arseniyadis, Org. Lett. 2020, 22, 5995. doi: 10.1021/acs.orglett.0c02079.问题 2
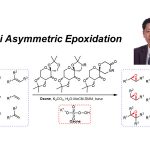
基本文献
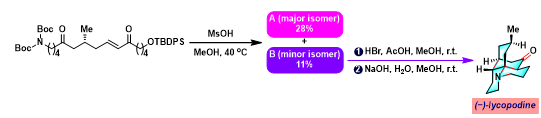
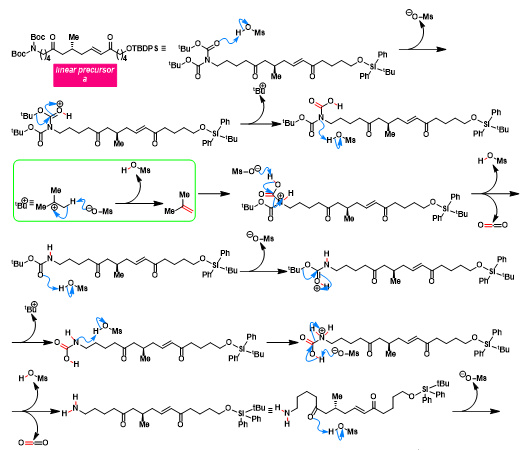
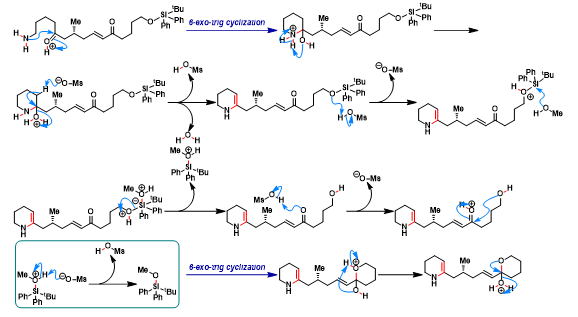
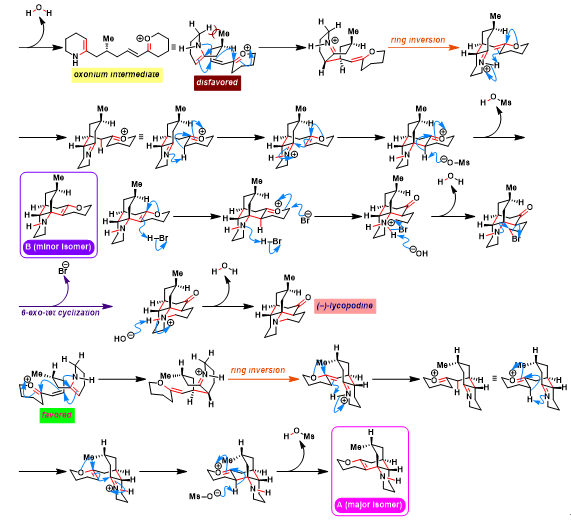
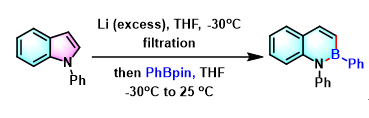
[1] K. Wada, N. Kogure, M. Kitajima, Takayama, Tetrahedron Lett. 2019, 60, 187. doi: 10.1016/j.tetlet.2018.12.007.反应机理
参考文献
[1] M. Azuma, T. Yoshikawa, N. Kogure, M. Kitajima, H. Takayama, J. Am. Chem.Soc. 2014, 136, 11618. doi: 10.1021/ja507016g.
[2] H. Takayama, J. Syn. Org. Chem. Jpn. 2015, 73, 1072. doi: 10.5059/yukigoseikyokaishi.73.1072. [3] A. Nakayama, N. Kogure, M. Kitajima, H. Takayama, Angew. Chem. Int. Ed. 2011, 50, 8025. doi: 10.1002/anie.201103550. [4] A. Nakayama, M. Kitajima, H. Takayama, Synlett 2012, 23, 2014. doi: 10.1055/s-0032-1316680. [5] H. Yang, R. G. Carter, L. N. Zakharov, J. Am. Chem. Soc. 2008, 130, 9238. doi: 10.1021/ja803613w. [6] H. Yang, R. G. Carter, J. Org. Chem. 2010, 75, 4929. doi: 10.1021/jo100916x. [7] D. A. Evans, J. R. Scheerer, Angew. Chem. Int. Ed. 2005, 44, 6038. doi: 10.1002/anie.200502296.问题 3
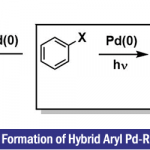
基本文献
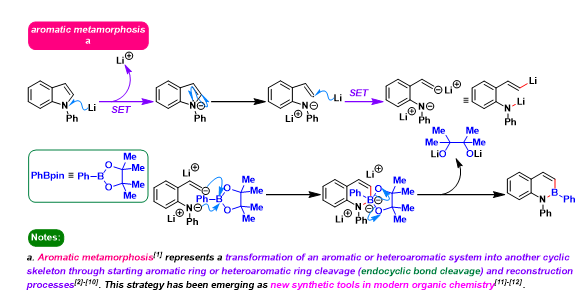
[1] S. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett..2019, 21, 10, 3855. doi: 10.1021/acs.orglett.9b01353.相关反应链接
Yorimitsu aromatic metamorphosis reaction
反应机理
参考文献
[1] D. Vasu, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2015, 54, 7162. doi: 10.1002/anie.201501992. [2] S. HayateK. Nogi, H. Yormitsu, Chem. Lett. 2017, 46, 1122. doi: 10.1246/cl.170415. [3] M. Bhanuchandra, K. Murakami, D. Vasu, H. Yorimitsu, A. Osuka, Angew.Chem. Int. Ed. 2015, 54, 10234. doi: 10.1002/anie.201503671.
[4] M. Bhanuchandra, H. Yorimitsu, A. Osuka, Org. Lett. 2016, 18, 384. doi: 10.1021/acs.orglett.5b03384. [5] M. Onoda, Y. Koyanagi, H. Saito, M. Bhanuchandra, Y. Matano, H. Yorimitsu,Asian J. Org. Chem. 2017, 6, 257. doi: 10.1002/ajoc.201600612.
[6] S. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett. 2017, 19, 5557. doi: 10.1021/acs.orglett.7b02660. [7] H. Saito, S. Otsuka, K. Nogi, H. Yorimitsu, J. Am. Chem. Soc. 2016, 138, [8] Y. Kurata, S. Otsuka, N. Fukui, K. Nogi, H. Yorimitsu, A. Osuka, Org. Lett. 2017, 19, 1274. doi: 10.1021/acs.orglett.6b03861. [9] S. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett. 2019, 21, 3855. doi: 10.1021/acs.orglett.9b01353. [10] Y. Zhou, L. Lin,Y. Wang, J. Zhu, Q. Song, Org. Lett. 2019, 21, 7630. doi: 10.1021/acs.orglett.9b02933. [11] H. Yorimitsu, D. Vasu, M. Bhanuchandra, K. Murakami, A. Osuka, Synlett2016, 27, 1765. doi: 10.1055/s-0035-1561617.
[12] K. Nogi, H. Yorimitsu, Chem. Commun. 2017, 53, 4055. doi: 10.1039/C7CC00078B.







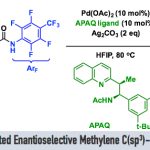








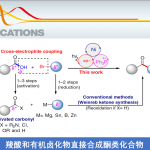

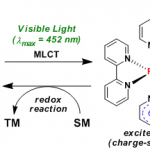

No comments yet.