作者:Asymmboy
概要
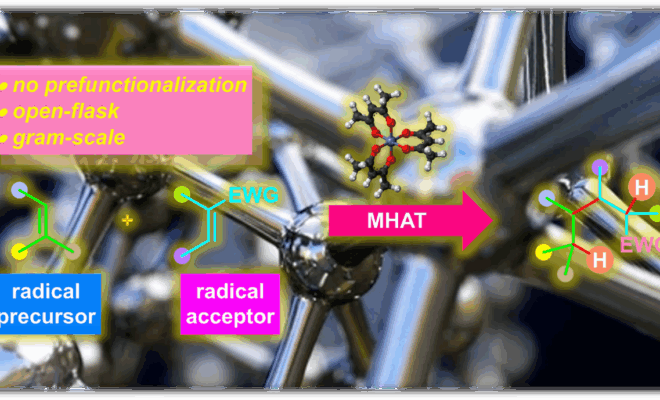
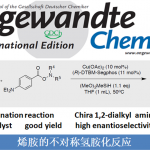
BROC (Baran reductive olefin coupling) 反应是在铁(III)催化剂与有机硅烷还原剂存在下,通过MHAT (metal-hydride hydrogen atom transfer) 活化过程进行的电中性烯基化合物、供电子烯基化合物与缺单子烯基化合物之间的还原偶联反应。该反应由美国TSRI的P. S. Baran团队在2014年首次报道[1]-[4]。BROC反应具有反应条件较为温和,操作简便,对于氧气及湿气较不敏感,广泛的底物应用范围,优良的官能团兼容性与氧化还原经济性以及潜在的放大合成 (scale-up synthesis or large-scale synthesis) 应用前景等优势。目前已经广泛应用于各类具有立体阻碍的双环体系[1]-[4]、邻近的四级碳中心[1]-[4]、环丙烷环[1]-[4]以及部分天然产物分子[2], [5]-[7]的构建。
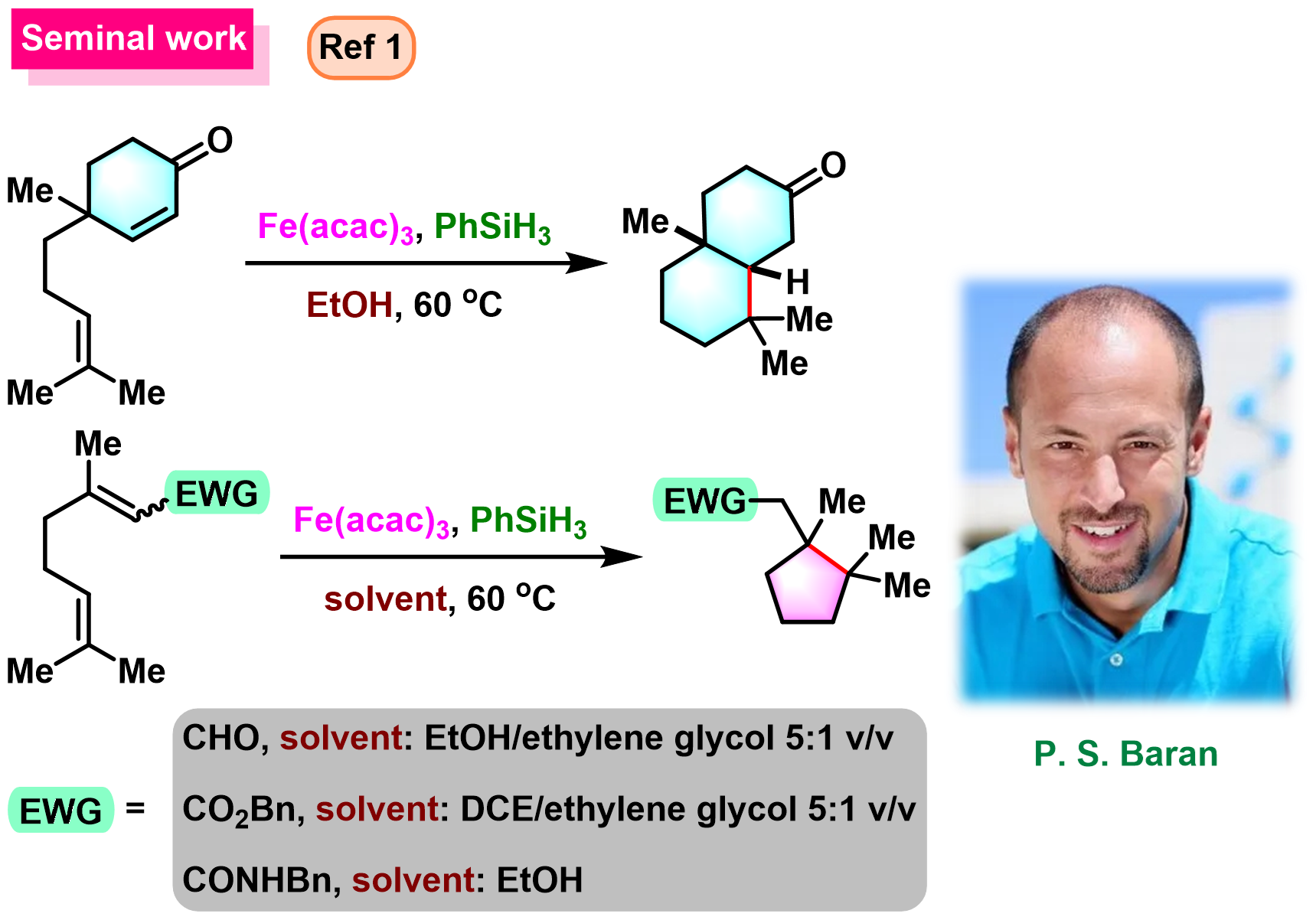
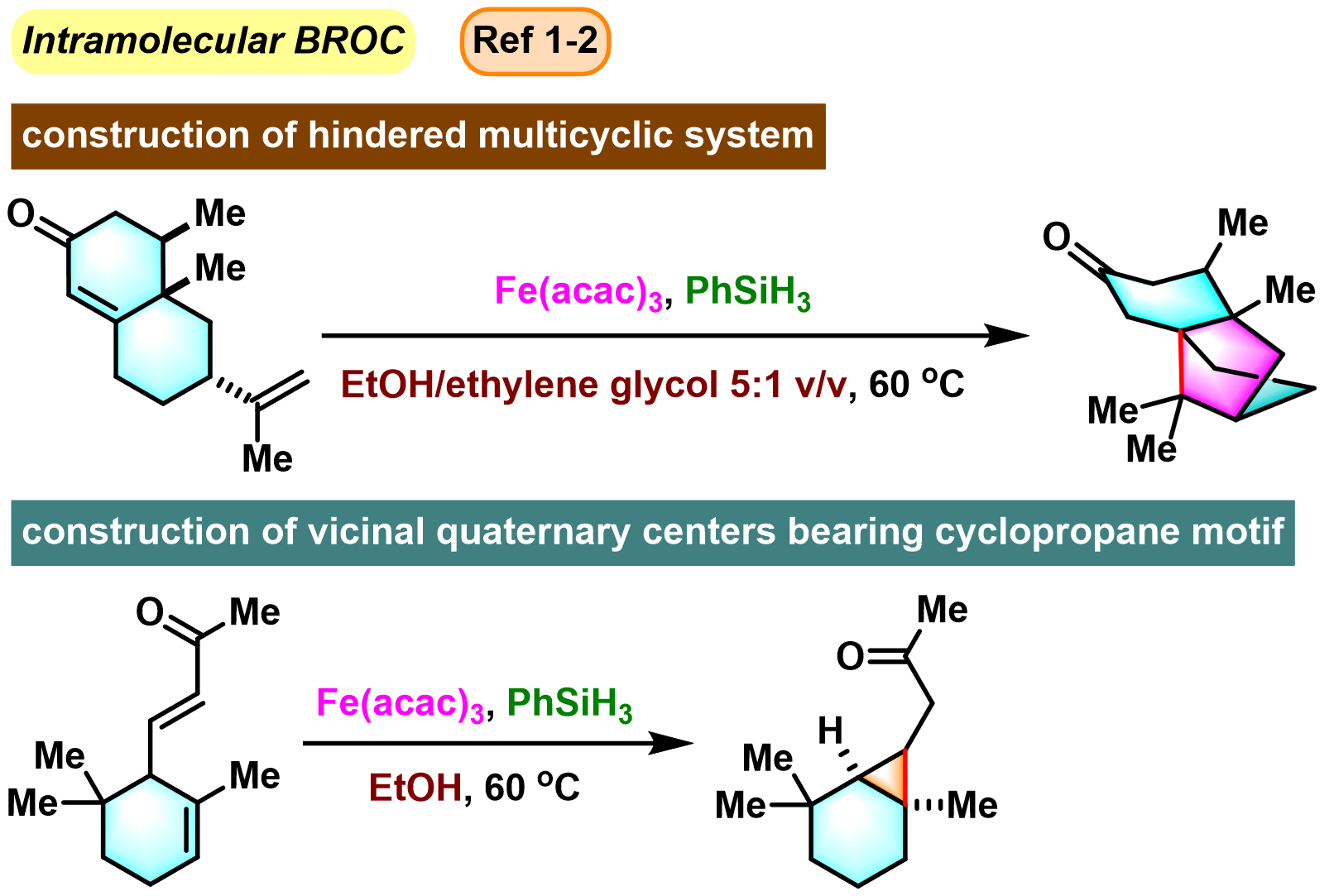
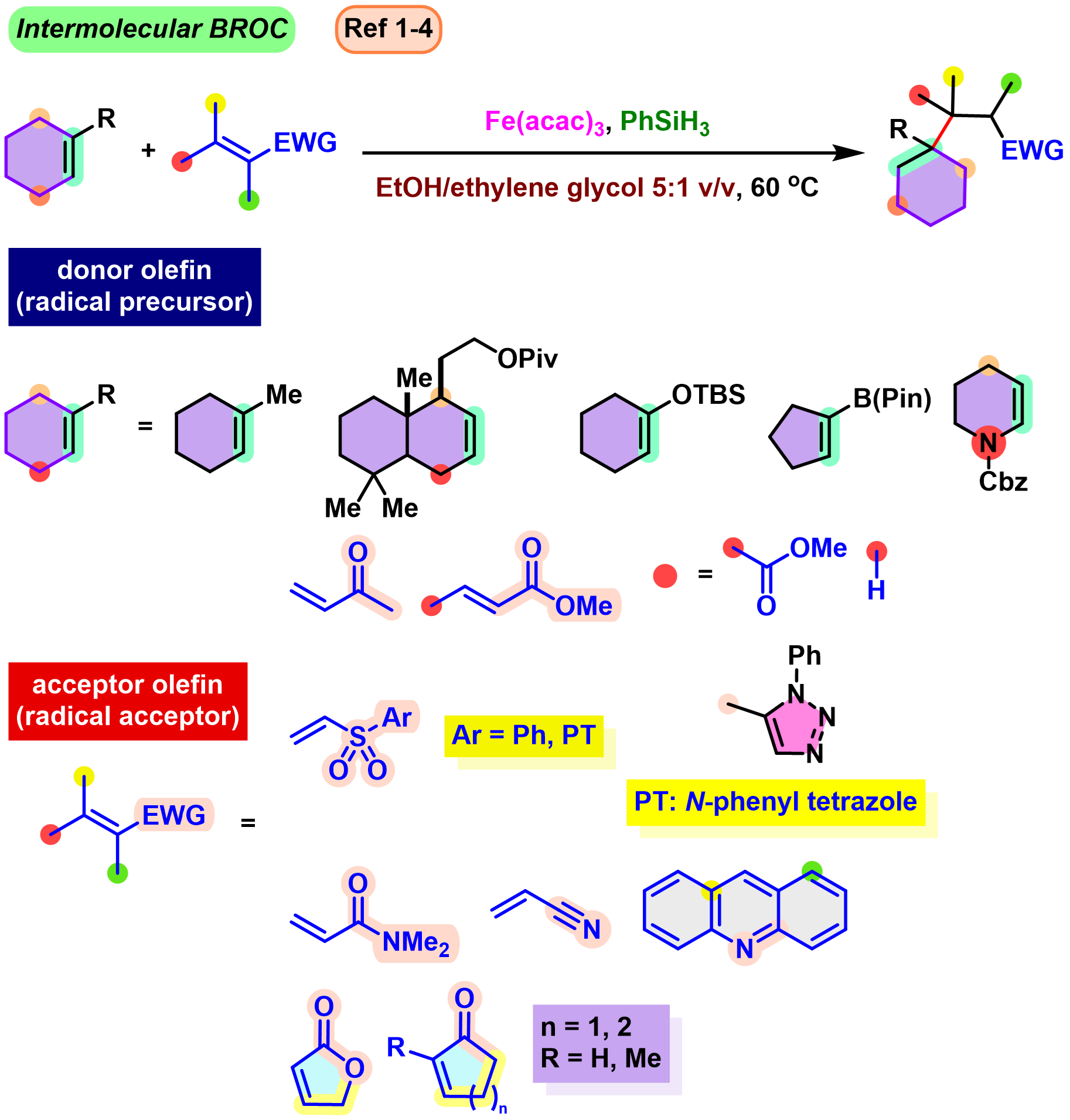
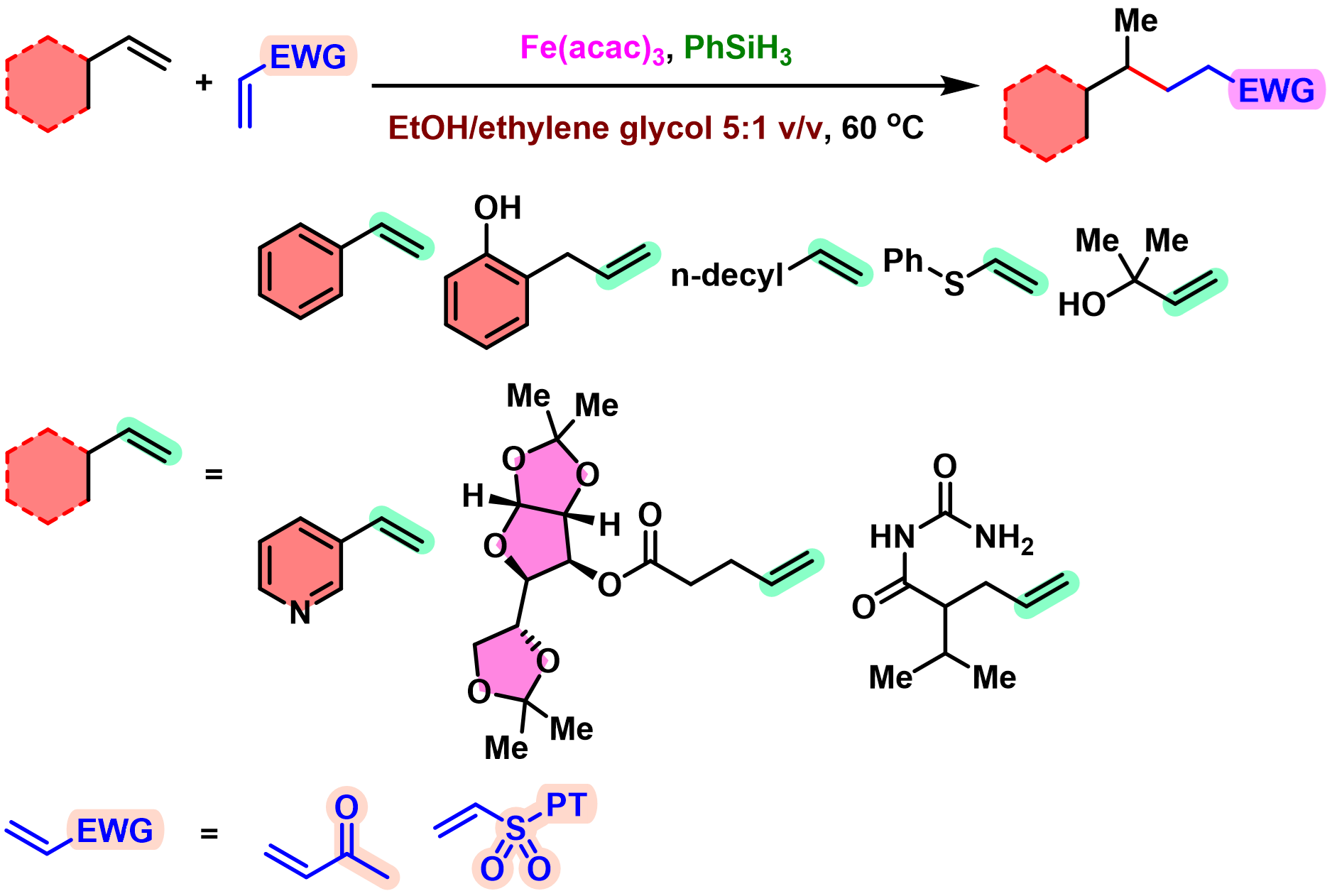
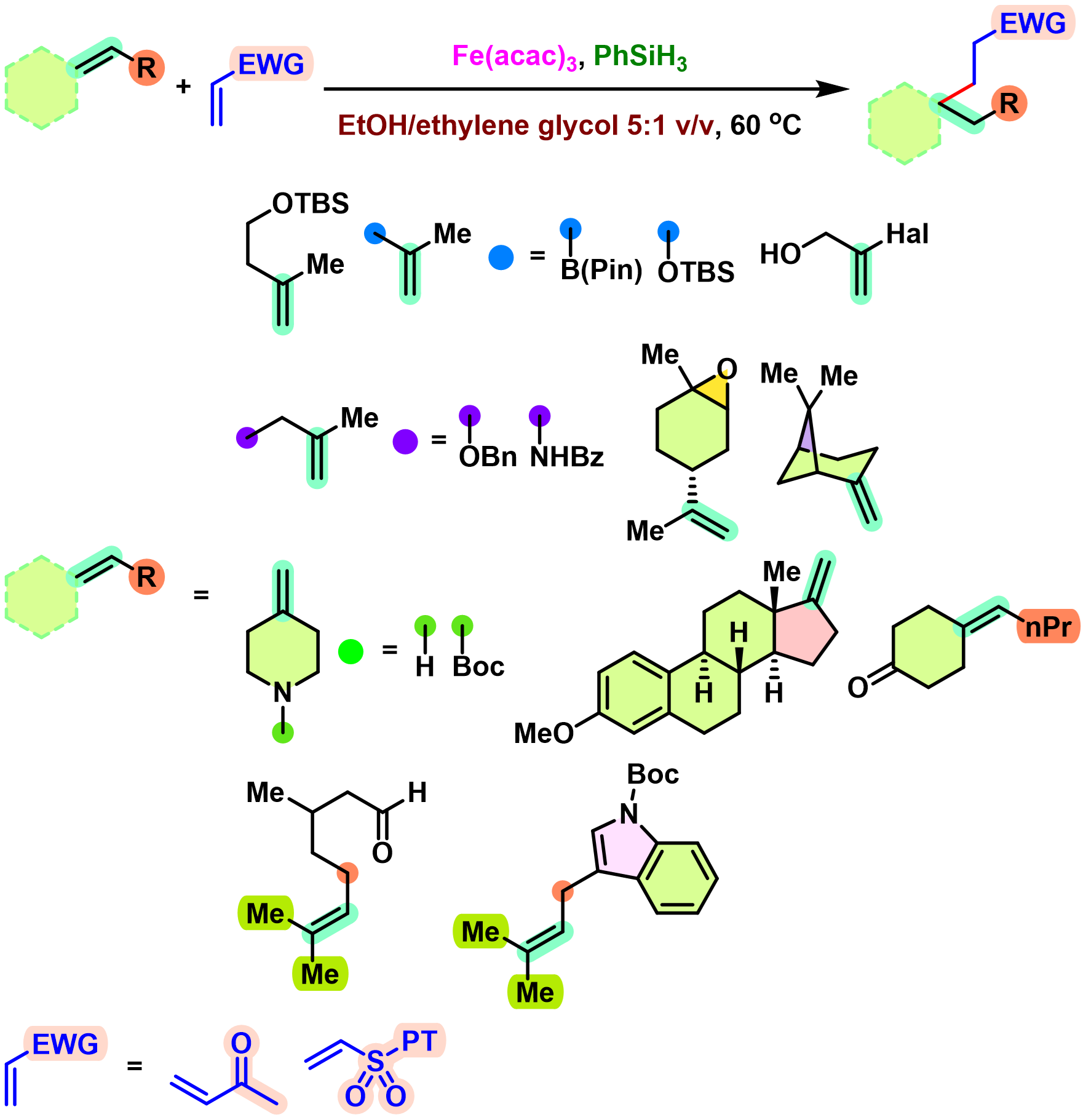
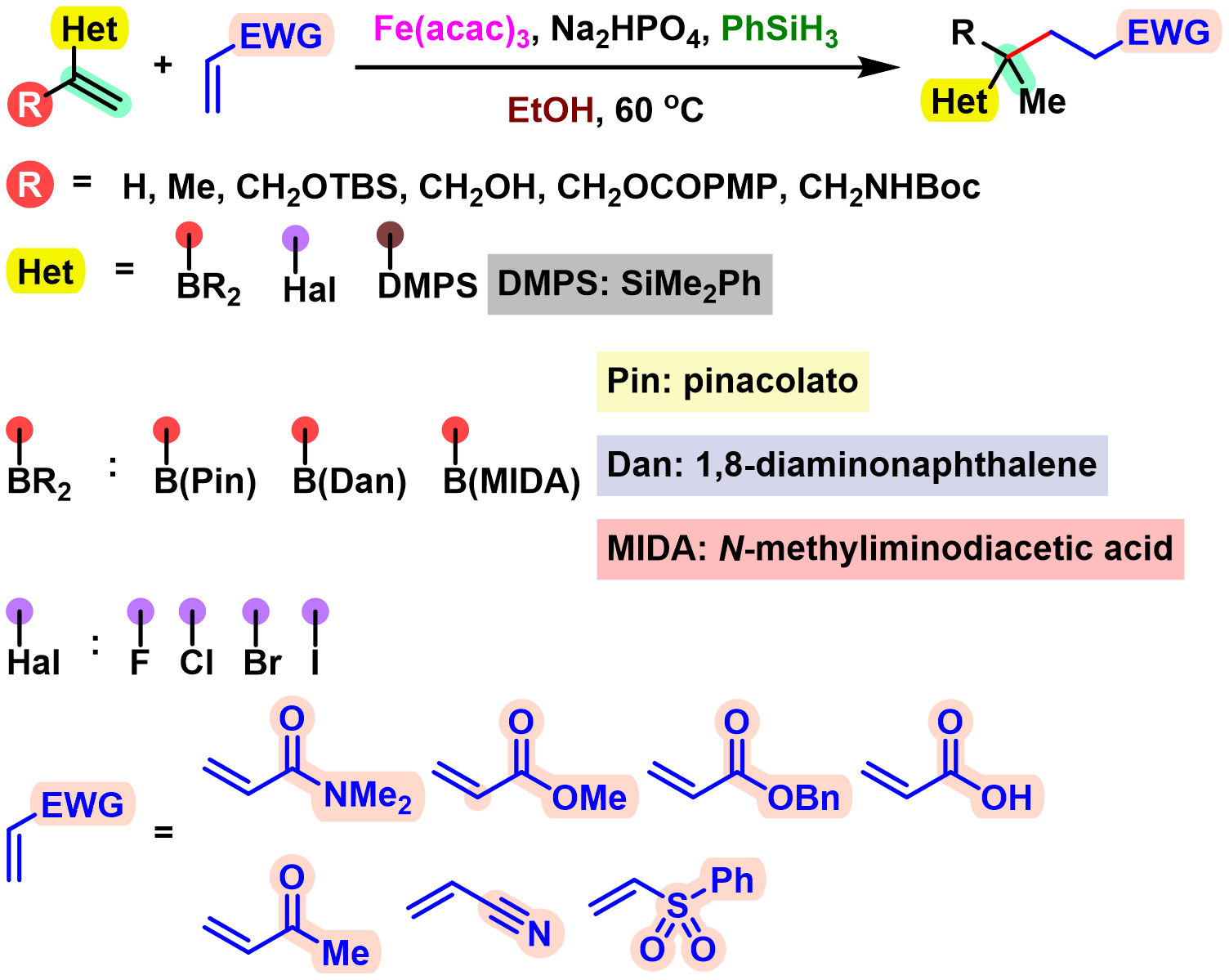
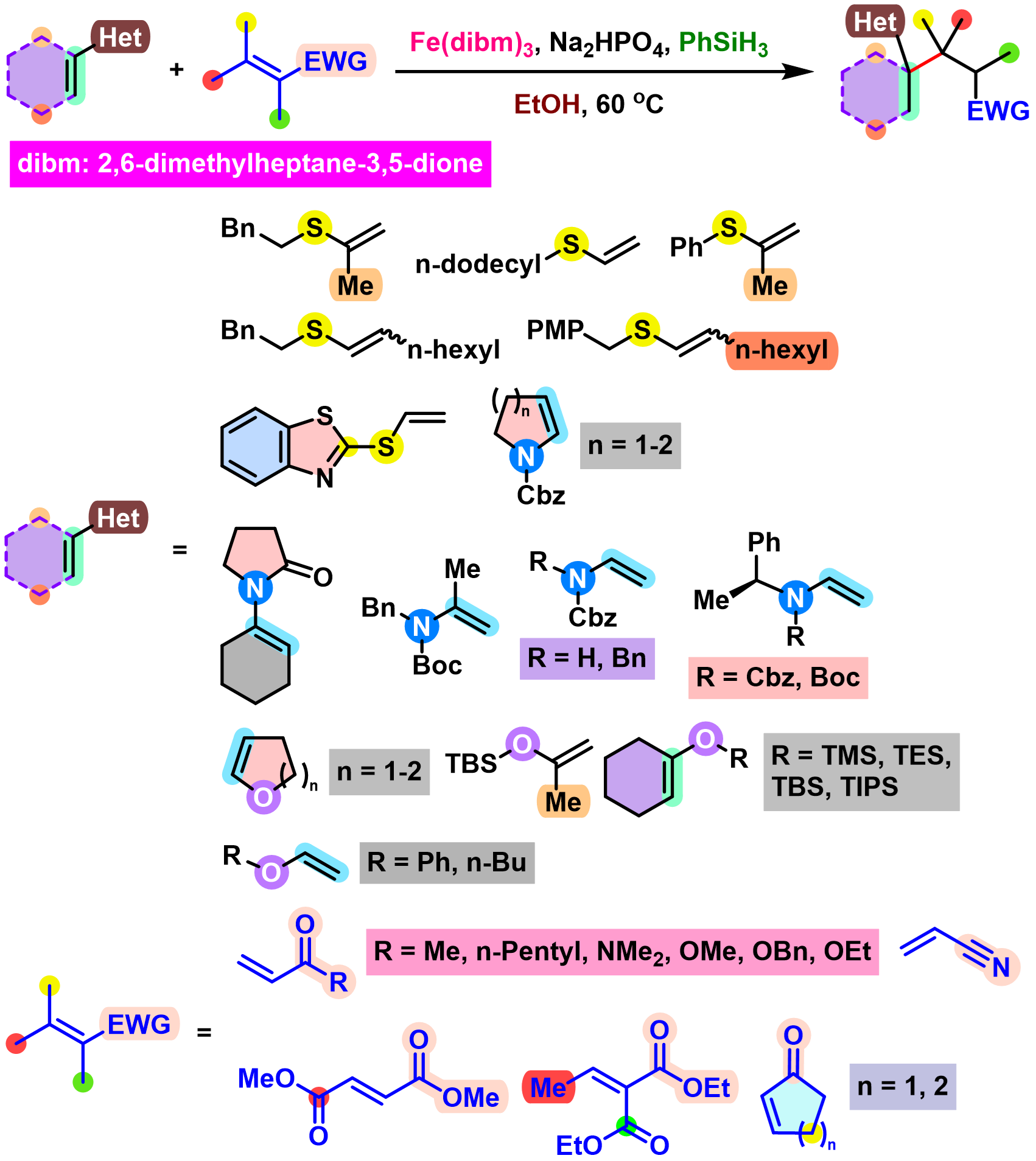
基本文献
[1] J. C. Lo, L. Gui, Y. Yabe, C.-M. Pan, P. S. Baran, Nature 2014, 516, 343. doi: 10.1038/nature14006.
[2] G. L. Barnes, A. L. Rerick, P. S. Baran, Chem. Rxiv. 2025. Version 1. doi:
[3] J. C. Lo, Y. Yabe, P. S. Baran, J. Am. Chem. Soc. 2014, 136, 1304. doi: 10.1021/ja4117632.
[4] J. C. Lo, D. Kim, C.-M. Pan, J. T. Edwards, Y. Yabe, J. Gui, T. Qin, S. Gutiérrez, J. Giacoboni, M. W. Smith, P. L. Holland, P. S. Baran, J. Am. Chem. Soc. 2017, 139, 2484. doi: 10.1021/jacs.6b13155.
[5] G. Huang, X. Zhang, Y. Gu, J. Gui, J. Am. Chem. Soc. 2025, 147, 20239. doi: 10.1021/jacs.5c07053.
[6] J. Wu, J. Bao, J. Deng, H. Tian, J. Gui, J. Am. Chem. Soc. 2025, 147, 30599. doi: 10.1021/jacs.5c09579.
[7] S. W. M. Crossley, C. Obradors, R. M. Martinez, R. A. Shenvi, Chem. Rev. 2016, 116, 8912. doi: 10.1021/acs.chemrev.6b00334.
反应机理
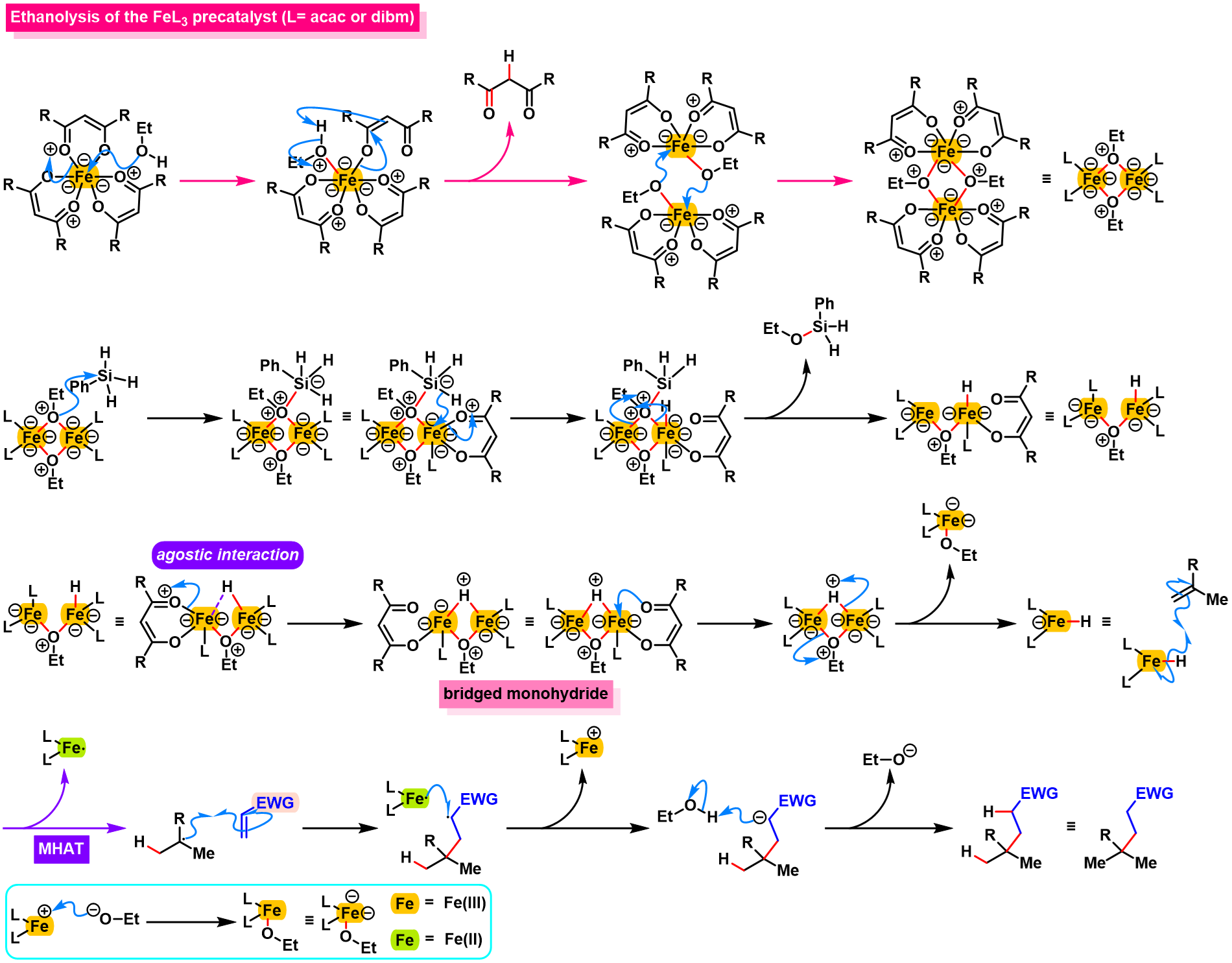
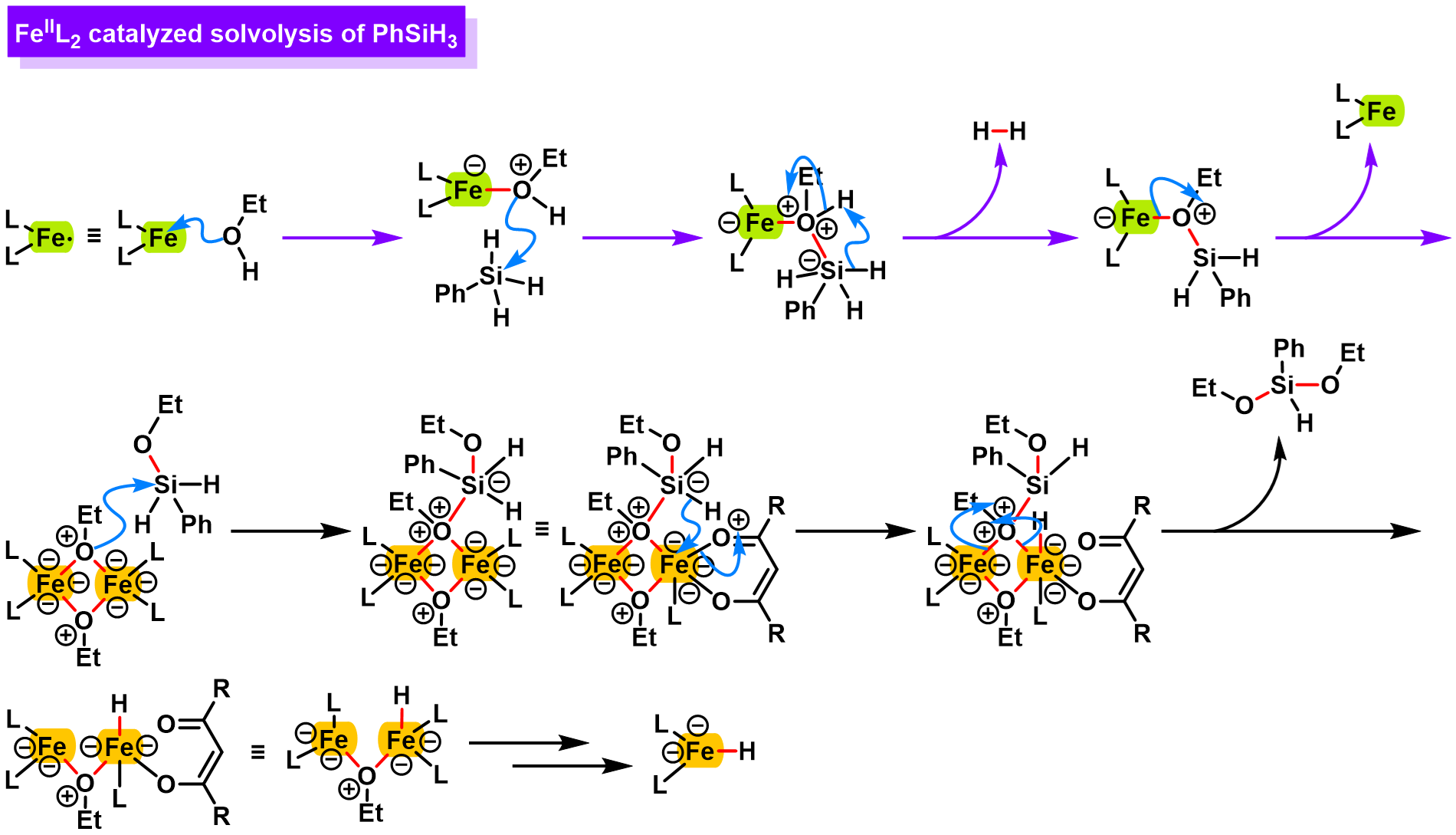
参考文献
Solvolysis of the FeL3 precatalyst:
[1] G. Gumbel, H. Elias, Inorg. Chim. Acta 2003, 342, 97. doi: 10.1016/S0020-1693(02)01143-X.
[2] C.-H. S. Wu, G. R. Rossman, H. B. Gray, G. S. Hammond, H. J. Schugar, Inorg. Chem. 1972, 11, 990. doi: 10.1021/ic50111a015.
FeL2-catalyzed solvolysis of PhSiH3:
[3] C. Obradors, R. M. Martinez, R. A. Shenvi, J. Am. Chem. Soc. 2016, 138, 4962. doi: 10.1021/jacs.6b02032.
MHAT:
[4] J. Wu, Z. Ma, Org. Chem. Front. 2021, 8, 7050. doi: 10.1039/D1QO01139A.
[5] S. W. M. Crossley, C. Obradors, R. M. Martinez, R. A. Shenvi, Chem. Rev. 2016, 116, 8912. doi: 10.1021/acs.chemrev.6b00334.
[6] S. A. Green, S. W. M. Crossley, J. L. M. Matos, S. Vasquez-Cespedes, S. L. Shevick, R. A. Shenvi, Acc. Chem. Res. 2018, 51, 2628. doi: 10.1021/acs.accounts.8b00337.
[7] L. G. Rodríguez, J. Bonjoch, B. Bradshaw, Org. Lett. 2024, 26, 10553. doi: 10.1021/acs.orglett.4c03943.
反应实例
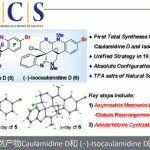
harziandione的全合成[1]
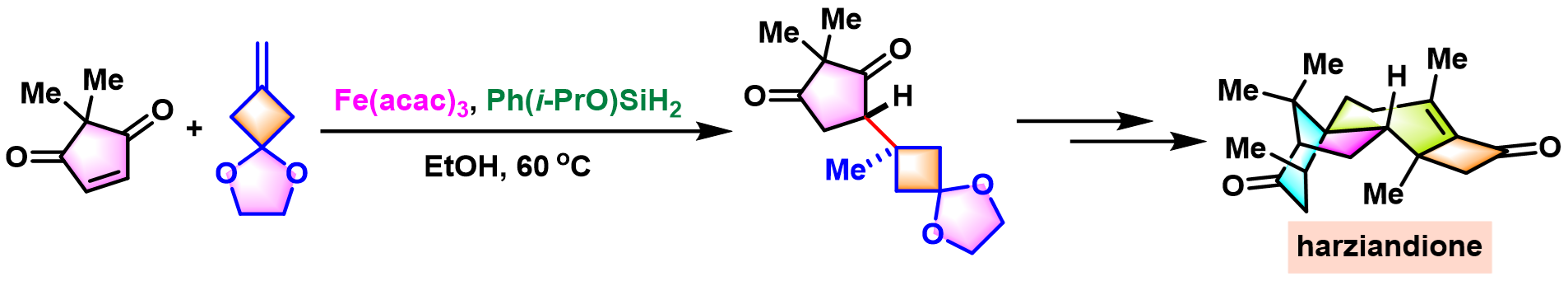
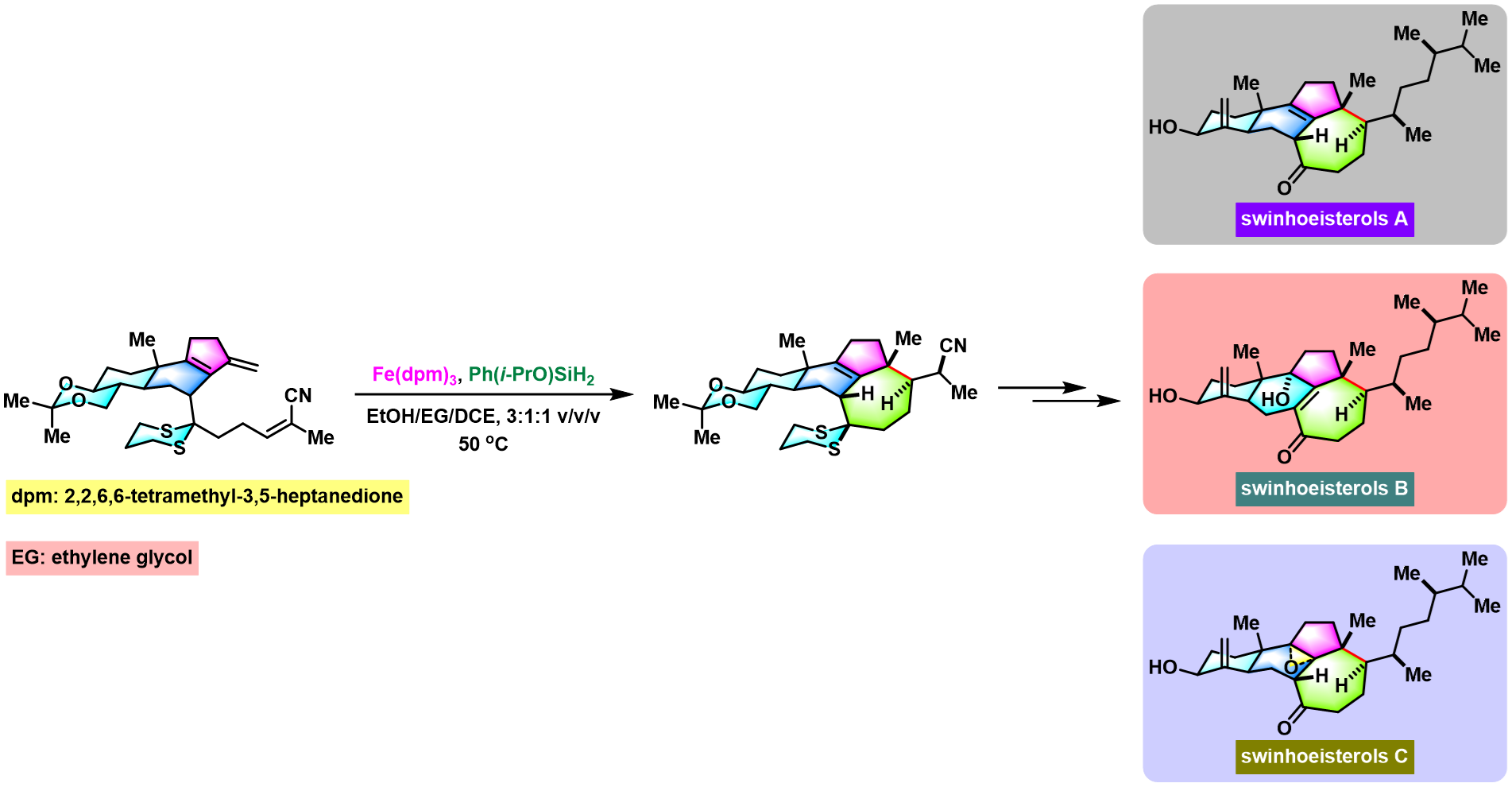
swinhoeisterols A – C的全合成[2]
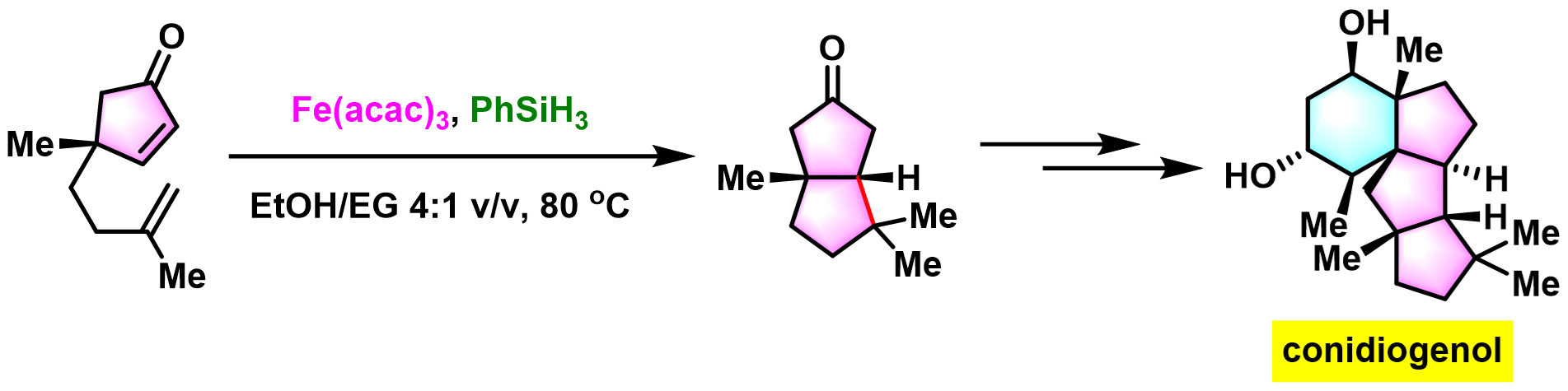
conidiogenol的全合成[3]
酚的合成[4]

烯基化合物的hydromethylation[5]
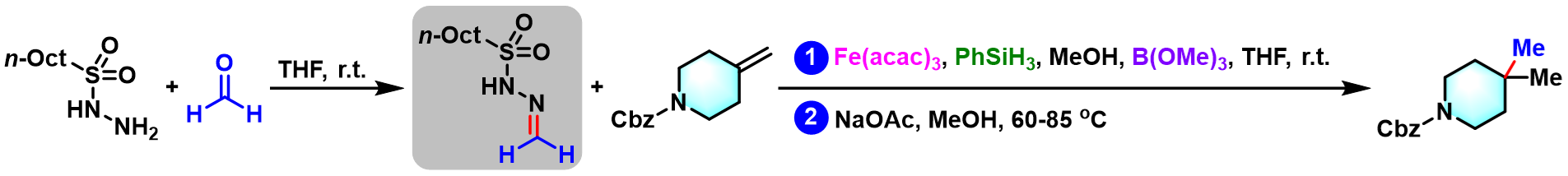
(±)-hippolachnin A的全合成[6]
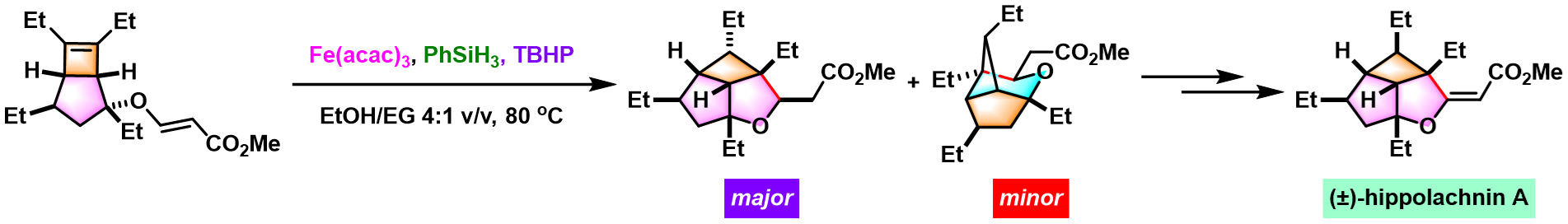
emindole SB的全合成[7]
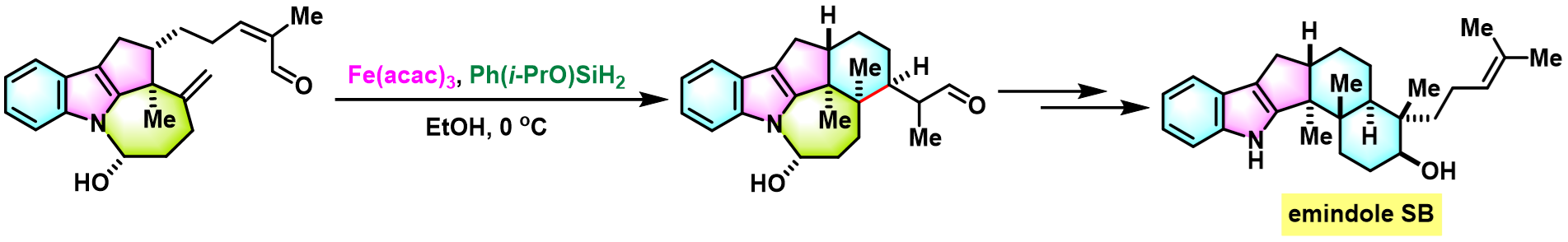
实验步骤

参考文献
[1] J. Wu, J. Bao, J. Deng, H. Tian, J. Gui, J. Am. Chem. Soc. 2025, 147, 30599. doi: 10.1021/jacs.5c09579.
[2] G. Huang, X. Zhang, Y. Gu, J. Gui, J. Am. Chem. Soc. 2025, 147, 20239. doi: 10.1021/jacs.5c07053.
[3] P. F. Hu, H. M. Chi, K. C. DeBacker, X. Gong, J. H. Keim, I. T. Hsu, S. A. Snyder, Nature, 2019, 569, 703. doi: 10.1038/s41586-019-1179-2.
[4] Y. Shen, J. Qi, Z. Mao, S. Cui, Org. Lett. 2016, 18, 2722. doi: 10.1021/acs.orglett.6b01173.
[5] H. T. Dao, C. Li, Q. Michaudel, B. D. Maxwell, P. S. Baran, J. Am. Chem. Soc. 2015, 137, 8046. doi: 10.1021/jacs.5b05144.
[6] S. A. Ruider, T. Sandmeier, E. M. Carreira, Angew. Chem. Int. Ed. 2015, 54, 2378. doi: 10.1002/anie.201410419.
[7] D. T. George, E. J. Kuenstner, S. V. Pronin, J. Am. Chem. Soc. 2015, 137, 15410. doi: 10.1021/jacs.5b11129.













No comments yet.