作者:石油醚
简介
Josep Cornella(1985年2月2日–):西班牙有机和无机化学家,现为德国马普所教授。
个人名言: “If you’re not passionate, you will quit very quickly.”
课题组主页:https://www.cornellab.com/aboutjc
教育背景及工作经历
2008 University of Barcelona, MSc
2012 Queen Mary University of London, PhD, Prof. Igor Larrosa
2012-2015 Institute of Chemical Research of Catalonia, Postdoc, Prof. Ruben Martin
2015-2017 The Scripps Research Institute, Postdoc, Prof. Prof. Phil S. Baran
2017- Max-Planck-Institut für Kohlenforschung.
获奖情况
2026 – Earl Muetterties Memorial Lectureship, University of California, Berkeley
2026 – Tetrahedron Young Investigator Award For Organic Synthesis
2025 – OMCOS 22 Prize
2025 – EuroJOC Lectureship Award, SMOS-12
2025 – Andrew S. Kende Distinguished Lectureship, University of Rochester
2025 – Gassman Lectureship, University of Minnesota
2024 – 50 Scientists that Inspire, Cell Press
2023 – Presidential Lecture The Scripps Research Institute
2023 – EuroJIC Lectureship Award, EUCOMC XXV
2023 – 22/23 Chemical Communications Emerging Investigator Lectureship
2023 – 2023 Young Investigator Award Group Leader RSEQ
2022 – SN10 Scientists to Watch, class 2022, Science News Journal
2022 – Padwa Lectureship, Columbia University (NY, USA)
2022 – Merck Organic Chemistry Lecturer, University of Illinois Urbana-Champaign
2022 – Organometallics Distinguished Author Award
2021 – Novartis Early Career Award 2021
2021 – Kyoto Rising Star Lectureship (MSD Life Science Foundation – Japan)
2021 – Heinz Maier-Leibnitz-Preis 2021 (Deutsche Forschungsgemeinschaft)
2020 – ORCHEM Nachwuchspreis 2020
2020 – Bayer Early Excellence in Science Award 2020
2020 – Ruhrpreis für Kunst und Wissenschaft 2020
2020 – Otto Röhm Gedächtnisstiftung Forschung Preis 2020
2020 – C&EN Talented 12 – Class 2020
2020 – Dozentenpreis des Fonds (Fonds der Chemischen Industrie)
2020 – Marcial Moreno Mañas Award (Real Sociedad Española de Quimica-Catalan Section)
2020 – ERC Starting Grant
2019 – Münster Symposium-CEC Young Researcher Award
2019 – Junior Scientist Program Fellowship (JSP – Bürgenstock)
2018 – Sachkostenzuschüsse Fonds der Chemischen Industrie
2018 – Thieme Chemistry Journals Award
2017 – Independent Max-Planck Research Group Leader
prior to MPI
2016 – TSRI Travel Award (Society of Fellows)
2015 – Beatriu de Pinos Postdoctoral Fellowship (Prof. Phil S. Baran)
2013 – Marie Skłodowska-Curie Fellowship (Prof. Ruben Martin)
2012 – COFUND Postdoc Fellowship (Prof. Ruben Martin)
研究概要
自2017年建组以来,以“Sustainable Catalysis Lab”为理念,致力于开发廉价的镍、铋等新型催化剂,以及利用(N2O)的催化反应
新型铋催化剂
1)BiIII/BiV催化循环[1]
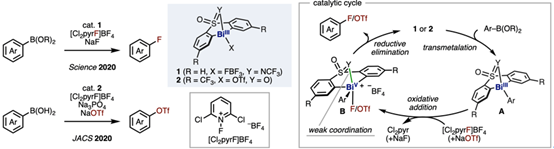
Josep Cornella教授建立了BiIII/BiV氧化还原催化循环,实现了芳基硼酸酯的氟化和三氟甲磺酰化。即铋催化剂1、2,6-二氯-1-氟吡啶盐([Cl2pyrF]BF4)和NaF体系下实现了芳基硼酸酯的氟化。其反应机理:1与芳基硼酸酯经转金属化生成芳基BiIII中间体A。随后,[Cl2pyrF]BF4对A发生氧化加成生成阳离子型BiV中间体B,再经还原消除得到氟代芳烃并再生催化剂1。该循环的关键在于:(1)采用易于发生氧化加成的氟吡啶盐;(2)配体中磺酰亚胺的氮原子弱配位稳定阳离子型BiV中间体B。此外,BiIII催化剂2与NaOTf作用下,还可实现芳基硼酸酯的三氟甲磺酰化。
2)BiI/BiI催化循环[2, 3]
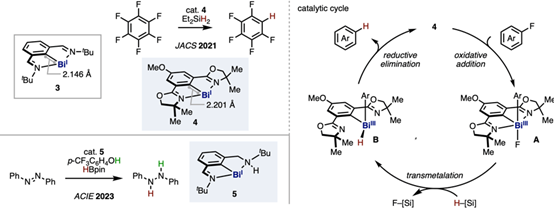
BiI物种易氧化且不稳定,难以用于催化反应。前人研究发现,一种基于N,C,N-钳形配体的铋宾(bismutinidene)络合物3以单体形式存在,据此推测,若采用类似结构的催化剂,有望建立BiI/BiIII的常规催化体系。在实际应用中,采用结构与3相似的Bi/Phebox催化剂4,通过二乙基硅烷成功实现全氟芳烃的氢化脱氟反应。其反应机理:4与全氟芳烃经氧化加成生成BiIII物种A,A与硅烷经转金属化生成BiIII络合物 B。最后,B经还原消除获得产物并再生催化剂4。进一步研究发现,3不能用于此反应。X射线晶体结构分析结果表明,4的C(sp2)-Bi键长比3长。此结果表明,铋中心的非键合电子对向邻近sp²碳原子的离域收到抑制,并改善了对BiI物种氧化加成的反应性。与此相关,已有文献报道,在BiI催化剂5存在下,可利用三氟甲基苯酚与频哪醇硼烷(HBpin)实现偶氮苯的还原反应。
3) BiI/BiII/BiIII循环[4]
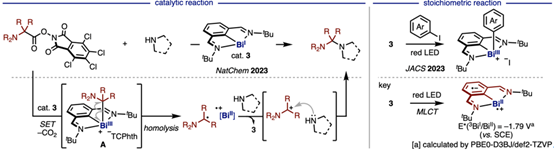
采用铋催化剂3,实现了氧化还原活性酯与四氢吡咯之间的C—N反应。其具体的反应机制如下,即3通过单电子转移(SET)对活性酯进行氧化加成,随后发生脱羧,并经自由基重组生成BiIII中间体A。中间体A的C—Bi键发生均裂,释放烷基自由基并生成BiII•+物种。在后续过程中,该烷基自由基被BiII•+氧化,进而与唑类发生偶联反应,同时再生催化剂3,从而完成BiI/BiII/BiIII的催化循环。
除上述体系外,铋催化剂还展现出其他独特反应特性。例如,在红光照射下,催化剂3与碘代芳烃发生氧化加成。该过程的关键在于光照诱导的金属到配体电荷转移(MLCT),即电子从BiI中心向配体转移,从而生成具有强还原能力的BiII•+活性物种。
N2O作为氧源实现氧原子导入反应[5]
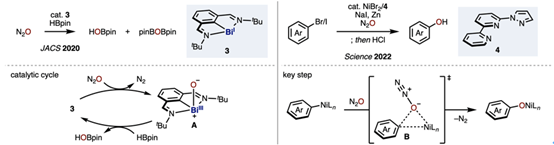
一氧化二氮(N2O)是一种具有显著温室效应的有害气体,其全球增温潜势超过二氧化碳的300倍。近年来,已有研究提出将N2O作为潜在氧源加以资源化利用的策略。例如,在铋催化剂3的存在下,于N2O气氛中与HBpin发生反应,可实现氧原子对H—B键的插入,从而生成HOBpin。该过程可能通过催化剂3与N2O作用生成BiIII–oxo中间体A,随后被HBpin还原并释放氧原子的机理进行。此外,研究还发现,在含有自主设计的三齿配体4的镍催化体系中,结合NaI与锌还原剂,可实现由溴代或碘代烯烃出发合成酚类化合物。此转化可能涉及烷基镍物种与N2O反应,经由类似Baeyer–Villiger氧化的过渡态B,实现氧原子对烷基–镍键的插入。
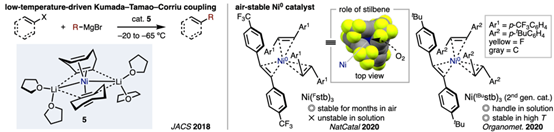
低价镍–烯烃络合物的性质解析与催化功能发现[6]
Josep Cornella教授发现了低价镍和烯烃配体组合的新型催化剂。如催化剂5在极低的温度下实现Kumada coupling。另外一方面,研究发现,含三个1,2-bis(4-(trifluoromethyl)phenyl)ethene配位的零价镍络合物(Ni(Fstb)3)可在空气中可稳定存在数天。X射线晶体结构分析表明,该稳定性源于苯乙烯配体以垂直于镍中心配位平面的方式排列,从而有效阻碍了氧分子的接近。尽管该催化剂具有优异的空气稳定性,其在现有反应中的催化活性仍可与Ni(cod)2相媲美。此外,将三氟甲基(CF3)替换为叔丁基(tBu)所得的第二代催化剂Ni(tBustb)3在溶液状态及高温条件下亦表现出良好的稳定性。上述试剂目前已由Strem Chemicals公司商业化供应。
吡喃鎓盐等杂环化合物的催化转化[7]
Josep Cornella教授已开发出多种针对杂环化合物官能团化的方法。例如,自主开发的吡啶盐试剂(Pyry·BF4)能够在多种亲核试剂共存的条件下,实现杂环上氨基的化学选择性转化,生成相应的吡啶衍生物,并在一锅反应中高效构建C–S、C–N和C–O键,同时实现氯化与羟基化等转化。该试剂目前已由Aldrich公司商业化供应。此外,在由卤代芳烃经两步合成的联苯二甲酰亚胺体系中,通过四氯异氰脲酸(TCICA)与KF协同作用,成功实现了含氟硫杂环化合物的多样性合成。
参考文献:
- (a) Planas, O.; Wang, F.; Leutzsch, M.; Cornella, J. Fluorination of Arylboronic Esters Enabled by Bismuth Redox Catalysis. Science 2020, 367, 313–317. DOI: 10.1126/science.aaz2258 (b) Planas, O.; Peciukenas, V.; Cornella, J. Bismuth-Catalyzed Oxidative Coupling of Arylboronic Acids with Triflate and Nonaflate Salts. J. Am. Chem. Soc. 2020, 142, 11382–11387. DOI: 10.1021/jacs.0c05343
- (a) Wang, F.; Planas, O.; Cornella, J. Bi(I)-Catalyzed Transfer-Hydrogenation with Ammonia-Borane. J. Am. Chem. Soc. 2019, 141, 4235–4240. DOI: 10.1021/jacs.9b00594 (b) Pang, Y.; Leutzsch, M.; Nöthling, N.; Katzenburg, F.; Cornella, J. Catalytic Hydrodefluorination via Oxidative Addition, Ligand Metathesis, and Reductive Elimination at Bi(I)/Bi(III) Centers. J. Am. Chem. Soc. 2021, 143, 12487–12493. DOI: 10.1021/jacs.1c06735 (c) Moon, H. W.; Wang, F.; Bhattacharyya, K.; Planas, O.; Leutzsch, M.; Nöthling, N.; Auer, A. A.; Cornella, J. Mechanistic Studies on the Bismuth-Catalyzed Transfer Hydrogenation of Azoarenes. Angew. Chem., Int. Ed. 2023, 62, e202313578. DOI: 10.1002/anie.202313578
- Šimon, P.; Proft, F. de; Jambor, R.; Růžička, A.; Dostál, L. Monomeric Organoantimony(I) and Organobismuth(I) Compounds Stabilized by an NCN Chelating Ligand: Syntheses and Structures. Angew. Chem., Int. Ed. 2010, 49, 5468–5471. DOI: 10.1002/anie.201002209
- (a) Mato, M.; Spinnato, D.; Leutzsch, M.; Moon, H. W.; Reijerse, E. J.; Cornella, J. Bismuth Radical Catalysis in the Activation and Coupling of Redox-Active Electrophiles. Nat. Chem. 2023, 15, 1138–1145. DOI: 10.1038/s41557-023-01229-7 (b) Mato, M.; Bruzzese, P. C.; Takahashi, F.; Leutzsch, M.; Reijerse, E. J.; Schnegg, A.; Cornella, J. Oxidative Addition of Aryl Electrophiles into a Red-Light-Active Bismuthinidene. J. Am. Chem. Soc. 2023, 145, 18742–18747. DOI: 10.1021/jacs.3c06651 (c) Yang, X.; Reijerse, E. J.; Bhattacharyya, K.; Leutzsch, M.; Kochius, M.; Nöthling, N.; Busch, J.; Schnegg, A.; Auer, A. A.; Cornella, J. Radical Activation of N–H and O–H Bonds at Bismuth(II). J. Am. Chem. Soc. 2022, 144, 16535–16544. DOI: 10.1021/jacs.2c05882
- (a) Vaillant, F. L.; Calbet, A. M.; González-Pelayo, S.; Reijerse, E. J.; Ni, S.; Busch, J.; Cornella, J. Catalytic Synthesis of Phenols with Nitrous Oxide. Nature 2022, 604, 677–683. DOI: 10.1038/s41586-022-04516-4 (b) Pang, Y.; Leutzsch, M.; Nöthling, N.; Cornella, J. Catalytic Activation of N2O at a Low-Valent Bismuth Redox Platform. J. Am. Chem. Soc. 2020, 142, 19473–19479. DOI: 10.1021/jacs.0c10092 (c) Ni, S.; Cornella, J. Catalytic Hydroxylation of Arylthianthrenium Salts with Nitrous Oxide. Tetrahedron 2023, 145, 133602. DOI: 10.1016/j.tet.2023.133602
- (a) Nattmann, L.; Lutz, S.; Ortsack, P.; Goddard, R.; Cornella, J. A Highly Reduced Ni–Li–Olefin Complex for Catalytic Kumada–Corriu Cross-Couplings. J. Am. Chem. Soc. 2018, 140, 13628–13633. DOI: 10.1021/jacs.8b09849 (b) Nattmann, L.; Saeb, R.; Nöthling, N.; Cornella, J. An Air-Stable Binary Ni(0)–Olefin Catalyst. Nat. Catal. 2020, 3, 6–13. DOI: 10.1038/s41929-019-0392-6 (c) Nattmann, L.; Cornella, J. Ni(4-tBustb)3: A Robust 16-Electron Ni(0) Olefin Complex for Catalysis. Organometallics 2020, 39, 3295–3300. DOI: 10.1021/acs.organomet.0c00485 (d) Lutz, S.; Nattmann, L.; Nöthling, N.; Cornella, J. 16-Electron Nickel(0)-Olefin Complexes in Low-Temperature C(sp2)–C(sp3) Kumada Cross-Couplings. Organometallics 2021, 40, 2220–2230. DOI: 10.1021/acs.organomet.0c00775
- (a) Moser, D.; Duan, Y.; Wang, F.; Ma, Y.; O’Neill, M. J.; Cornella, J. Selective Functionalization of Aminoheterocycles by a Pyrylium Salt. Angew. Chem., Int. Ed. 2018, 57, 11035–11039. DOI: 10.1002/anie.201806271 (b) Ghiazza, C.; Faber, T.; Gómez-Palomino, A.; Cornella, J. Deaminative Chlorination of Aminoheterocycles. Nat. Chem. 2022, 14, 78–84. DOI: 10.1038/s41557-021-00812-0 (c) Ghiazza, C.; Wagner, L.; Fernández, S.; Leutzsch, M.; Cornella, J. Bio-Inspired Deaminative Hydroxylation of Aminoheterocycles and Electron-Deficient Anilines. Angew. Chem., Int. Ed. 2023, 62, e202212219. DOI: 10.1002/anie.202212219 (d) Wang, L.; Cornella, J. A Unified Strategy for Arylsulfur(VI) Fluorides from Aryl Halides: Access to Ar-SOF3 Compounds. Angew. Chem., Int. Ed. 2020, 59, 23510–23515. DOI: 10.1002/anie.202009699
其他链接:






















No comments yet.