概要
Ohshiro-Hirao还原 (Ohshiro-Hirao reduction)是在碱性条件 (三乙胺[1]-[2], [4]、叔丁醇钾[3]或乙醇钠[5])下,亚磷酸二烷基酯 (dialkylphosphite)参与的1,1-二溴代烯[1]-[2], [4]、1,1-二溴环丙烷以及1,1-二卤代环丙烷[1]-[3]的化学选择性、区域选择性与立体选择性的还原脱卤过程,获得单卤代环丙烷或烯基溴的反应[1]-[5]。该反应由日本Osaka大学工学部石油化学系 (大阪大学工学部石油化学科, Department of Petroleum Chemistry, Faculty of Engineering, Osaka University)的Hirao (平尾 俊一,Hirao Toshikazu)与Ohshiro (大城 芳樹,Ohshiro Yoshiki)在1981年首次报道[1]。
Ohshiro-Hirao还原反应条件温和,具有较高的产率优良的化学选择性、区域选择性及立体选择性等优点。目前,该反应已经开始应用于有机合成[6]及生理活性分子[7]与药物分子合成[8]中关键砌块的构建[6]-[8]。
基本文献
- [1] T. Hirao, T. Masunaga, Y. Ohshiro, T. Agawa, J. Org. Chem. 1981, 46, 3745. doi: 10.1021/jo00331a039.
- [2] T. Hirao, S. Kohno, Y. Ohshiro, T. Agawa, Bull. Chem. Soc. Jpn. 1983, 56, 1881. doi: 10.1246/bcsj.56.1881.
- [3] G. Meijs, I. R. Doyle, J. Org. Chem. 1985, 50, 3713. doi: 10.1021/jo00220a008.
- [4] A. Sahar, J. H. Christopher, W. Stephen, Tetrahedron Lett. 2000, 41, 3215. doi: 10.1016/S0040-4039(00)00353-1.
- [5] C. Kuang, H. Senboku, M. Tokuda, Tetrahedron, 2002, 58, 1491. doi: 10.1016/S0040-4020(02)00013-3.
- [6] R. Rossi, A. Carpita, V. Lippolis, Synth. Commun. 1991, 21, 333. doi: 10.1080/00397919108016755.
- [7] S. Abbas, C. J. Hayes, Synlett 1999, 1124. doi: 10.1055/s-1999-2767.
- [8] J. Alzeer, J. Chollet, I. Heinze-Krauss, C. Hubschwerlen, H. Matile, R. G. Ridley, J. Med. Chem. 2000, 43, 560. doi: 10.1021/jm990002y.
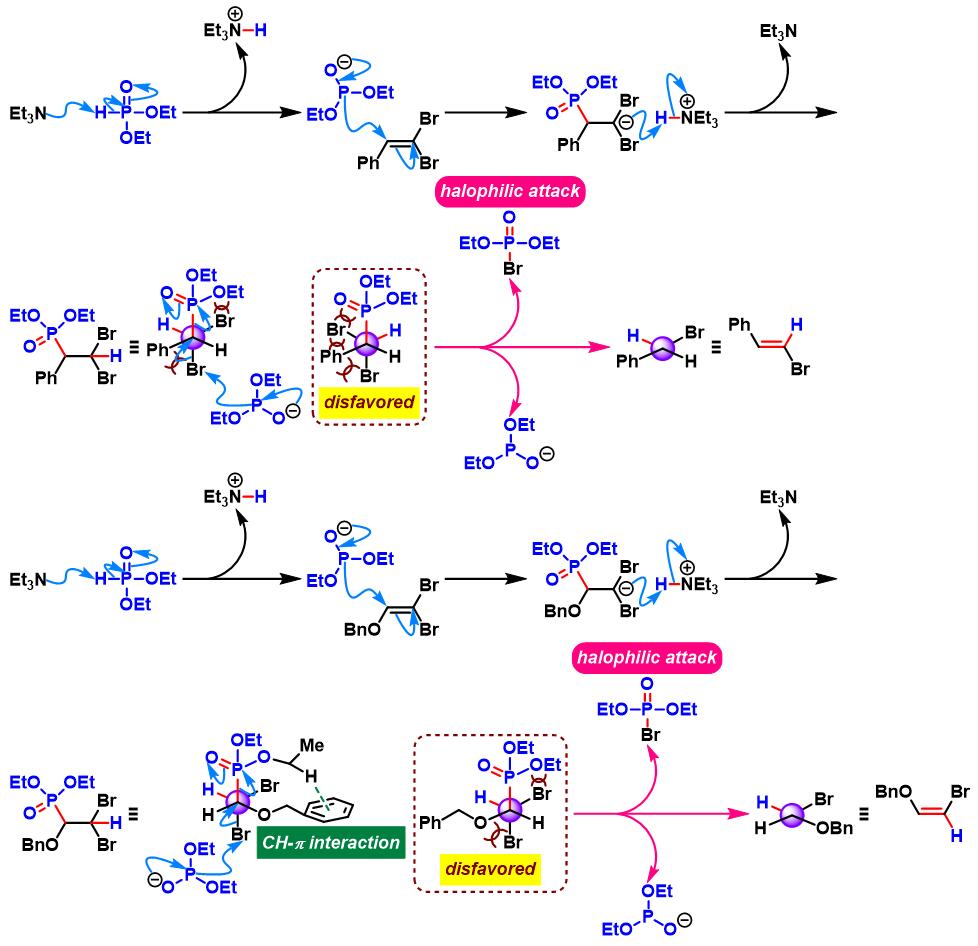
反应机理
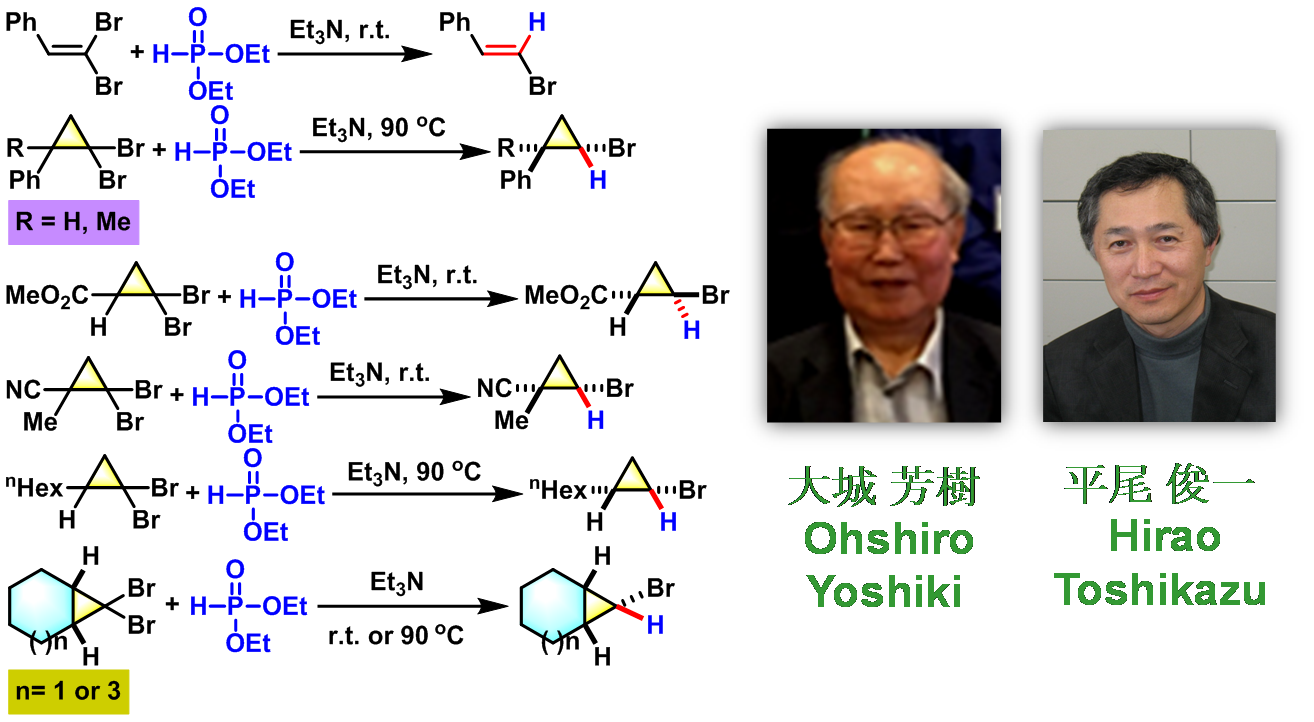
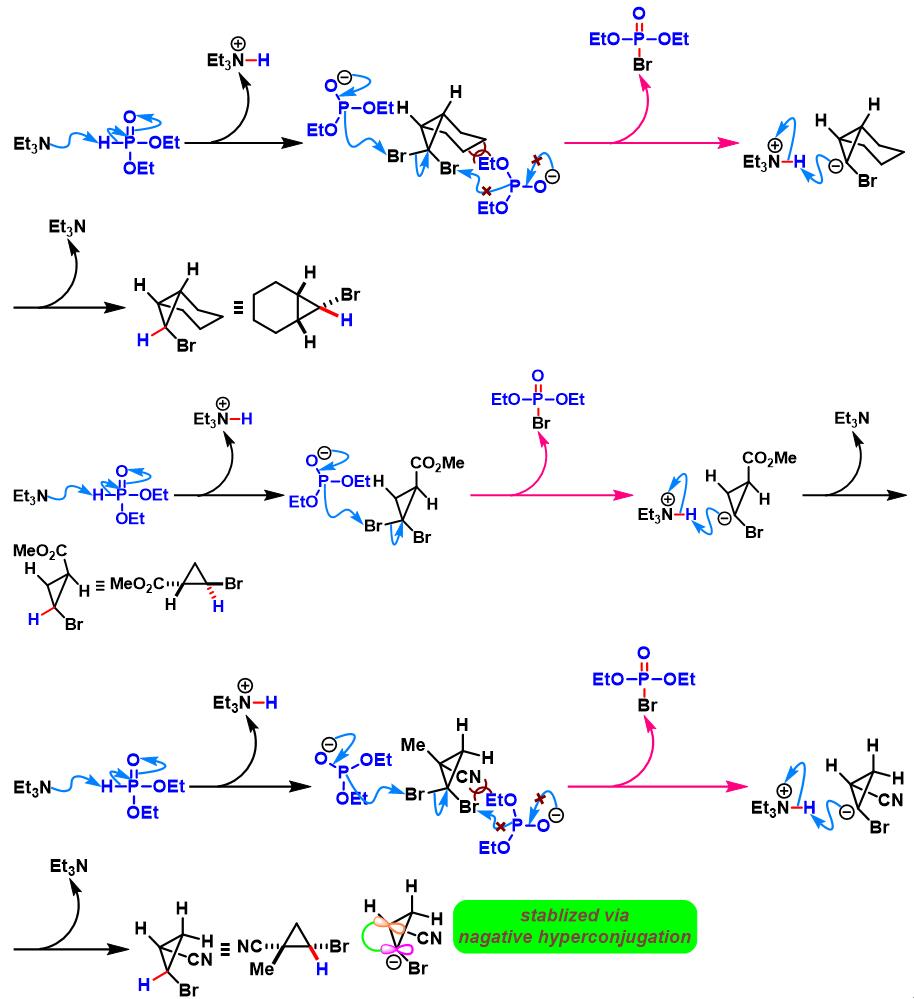
1. 三乙胺参与的Ohshiro-Hirao还原
(a) 1,1-二溴代烯的还原
(b) 1,1-二溴代环丙烷的还原
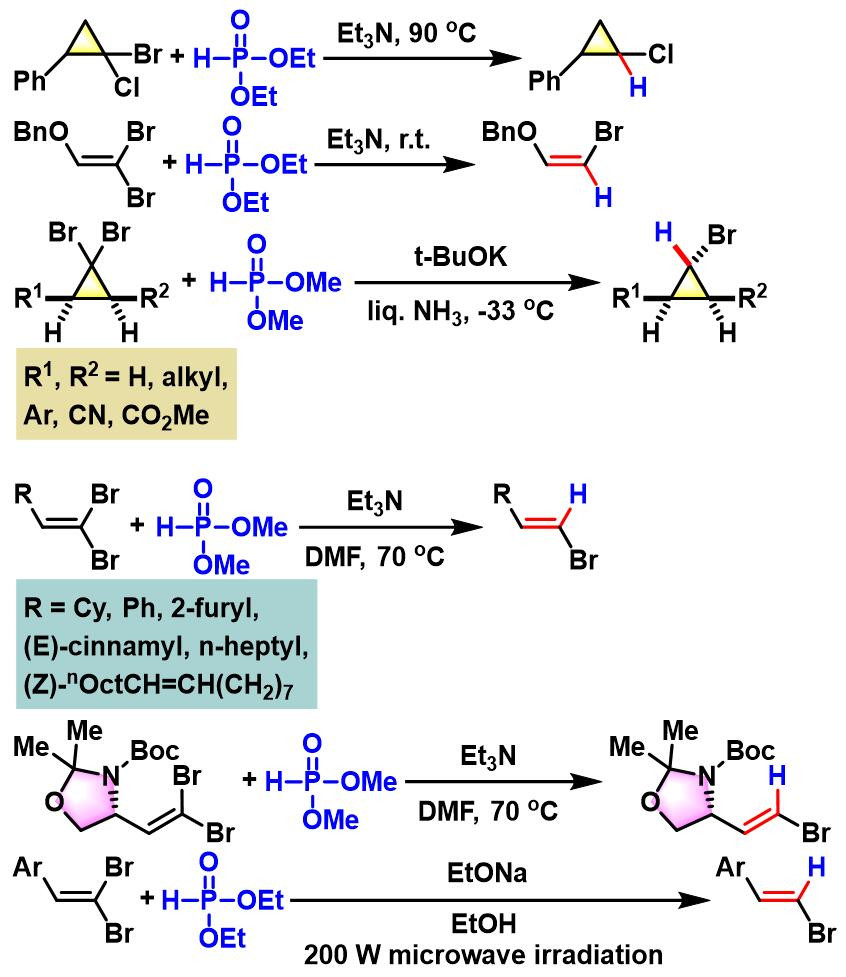
2. 乙醇钠参与的Ohshiro-Hirao还原
参考文献
- [1] A. Sahar, J. H. Christopher, W. Stephen, Tetrahedron Lett. 2000, 41, 3215. doi: 10.1016/S0040-4039(00)00353-1
- [2] G. Meijs, I. R. Doyle, J. Org. Chem. 1985, 50, 3713. doi: 10.1021/jo00220a008.
- [3] F. Ramirez, N. B. Desai, N. McKelvie, J. Am. Chem. Soc. 1962, 84, 1745. doi: 10.1021/ja00868a057.
- [4] N. S. Zefirov, D. I. Makhon’kov, Chem. Rev. 1982, 82, 615. doi: 10.1021/cr00052a004.
- [5] G. M. Steinberg, J. Org. Chem. 1950, 15, 637. doi: 10.1021/jo01149a031.
- [6] A. Kraszewski, J. Stawinski, Pure Appl. Chem. 2007, 79, 2217. doi: 10.1351/pac200779122217.
- [7] L. Liu,Y. Lv, Y. Wu,X. Gao, Z. Zeng,Y. Gao, G. Tang, Y. Zhao, RSC Adv. 2014, 4, 2322. doi: 10.1039/C3RA45212C.
- [8] G. Keglevich, R. Henyecz, Z. Mucsi, N. Z. Kiss, Adv. Synth. Catal. 2017, 359, 4322. doi: 10.1002/adsc.201700895.
反应实例
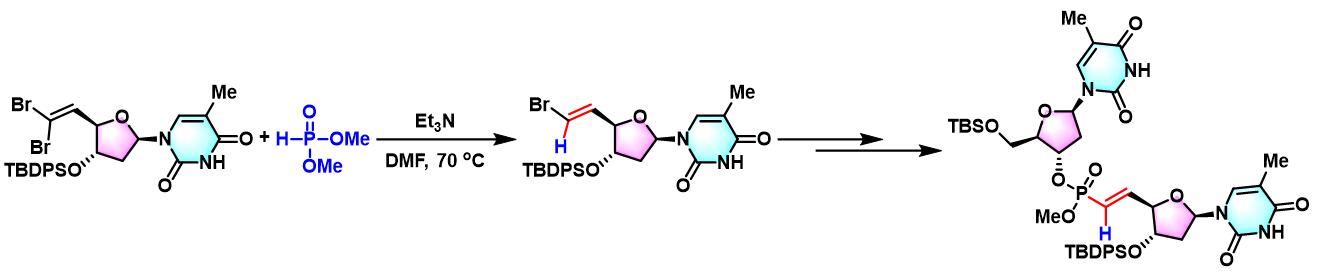
5ʹ-deoxy-5ʹ-methylidene phosphonate-containing thymidine二聚体的合成[1]-[2]
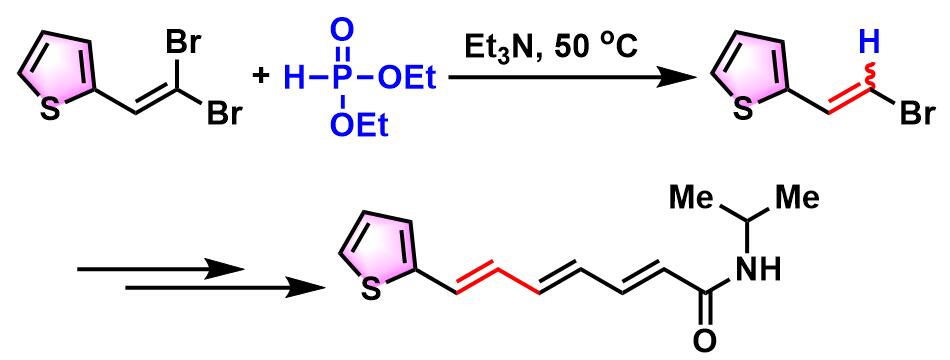
7-杂芳基(2E, 4E, 6E)-2, 4, 6-庚三烯酰胺的合成[3]
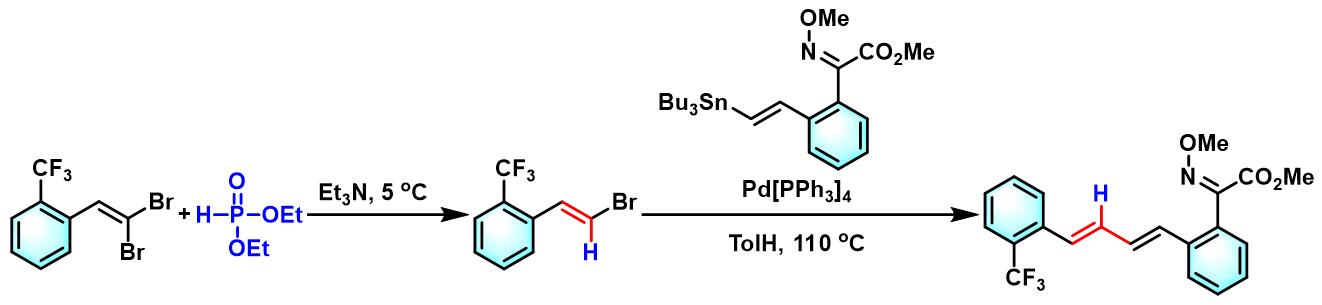
α-甲氧亚氨基乙酸酯的合成[4]
实验步骤
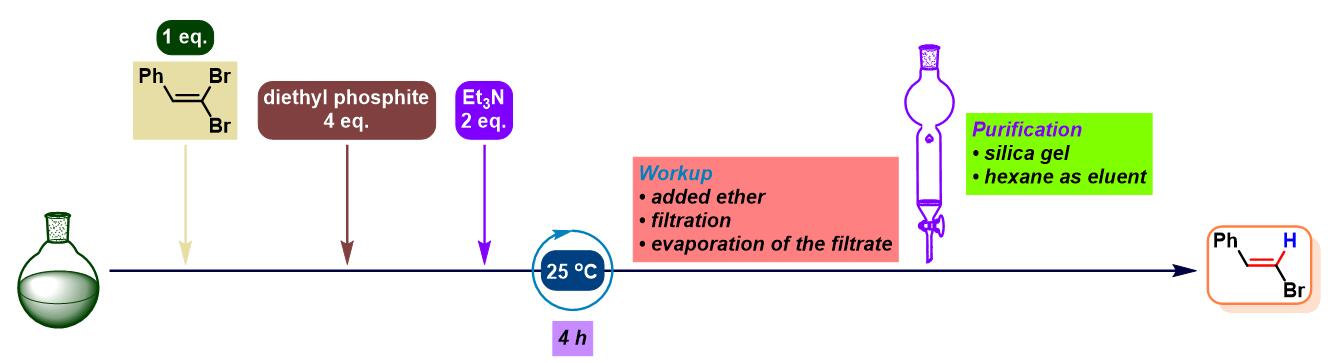
1. 三乙胺参与的Ohshiro-Hirao还原
(a) 1,1-二溴代烯的还原
(b) 1,1-二溴代环丙烷的还原
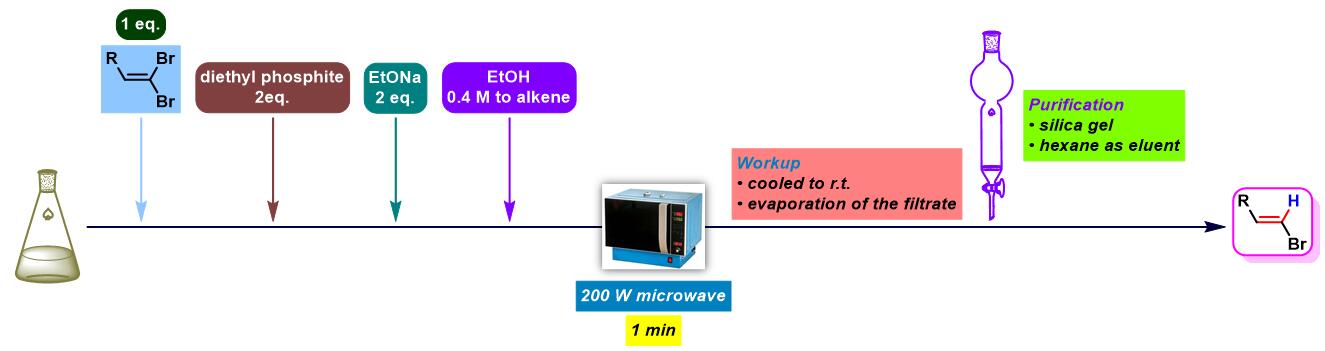
2. 乙醇钠参与的Ohshiro-Hirao还原
参考文献
- [1] A. Sahar, J. H. Christopher, W. Stephen, Tetrahedron Lett. 2000, 41, 3215. doi: 10.1016/S0040-4039(00)00353-1
- [2] S. Abbas, C. J. Hayes, Synlett 1999, 1124. doi: 10.1055/s-1999-2767.
- [3] R. Rossi, A. Carpita, V. Lippolis, Synth. Commun. 1991, 21, 333. doi: 10.1080/00397919108016755.
- [4] J. Alzeer, J. Chollet, I. Heinze-Krauss, C. Hubschwerlen, H. Matile, R. G. Ridley, J. Med. Chem. 2000, 43, 560. doi: 10.1021/jm990002y.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!

















No comments yet.