本文作者:alberto-caeiro
吉戒 直彦 Naohiko Yoshikai,日本有机化学家,师承日本东京大学中村 荣一教授,现为新加坡南洋理工大学物理与数学科学学院副教授。图片:实验室介绍。
经历
- 2000 B.Sc. Department of Chemistry, The University of Tokyo (Prof. Eiichi Nakamura)
- 2002 Visiting Student, Stockholm University (Prof. Jan-E. Bäckvall)
- 2005 Ph.D. Department of Chemistry, The University of Tokyo (Prof. Eiichi Nakamura)
- 2002.4-2005.2 JSPS Young Research Fellow
- 2005.2-2009.6 Assistant Professor, The University of Tokyo
- 2009.7-2016.8 Assistant Professor, Nanyang Technological University
- 2016.9-Present Associate Professor, Nanyang Technological University
获奖经历
- 2007 Inoue Research Award for Young Scientists
- 2008 Takasago Award, Society of Synthetic Organic Chemistry of Japan
- 2011 Thieme Chemistry Journals Award
- 2011 Asian Core Program Lectureship (Japan)
- 2014 SPMS Young Reseacher Award, NTU
- 2014 Asian Core Program Lectureship (China & Korea)
- 2014 Chemical Society of Japan Award for Young Chemists
- 2015 Asian Core Program Lectureship (Thailand)
工作介绍
1. Cobalt-catalyzed C-H bonds functionalization [1,2]
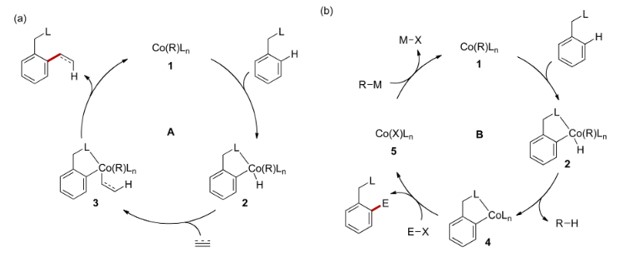
过渡金属催化的C–H键官能化反应一直是有机化学的研究热点之一。相较于此前的工作中使用的过渡金属为钯,铑等贵金属,Yoshikai教授则一直致力于廉价金属钴催化的C-H键官能团化反应。钴催化剂除了低成本外,在C-H键官能团化反应中具有独特的反应性和选择性。其反应主要包括以下几类:芳烃C-H键的烷基化{3},烯基化反应[4](烯烃,炔烃的氢芳基化反应);芳烃C-H键的亲电偶联反应[5]。
Co-Catalyzed C-H bond functionalization.
a: Hydroarylation of alkenes & alkynes; b: C-H bond electrophilic coupling
2. Migratory arylzincation
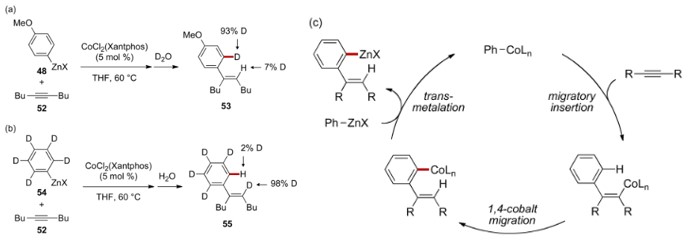
在Yoshikai教授研究C-H键官能团化反应中,他们在合成锌试剂时发现催化量的钴催化剂可加速锌插入芳基卤化物的速度[6]。在将制得的锌试剂直接与炔烃反应时,发现生成的产物是经过了[1,4]-钴迁移的芳基锌试剂,而非直接的插入产物烯基锌试剂,氘代实验也验证了该结果[7]。
Deuterium-labelling experiments & proposed mechanism involving 1,4-cobalt migration
3. Novel methodologies for Heterocycles synthesis
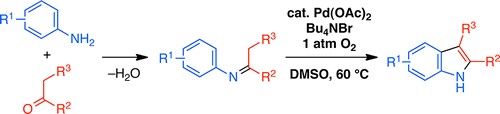
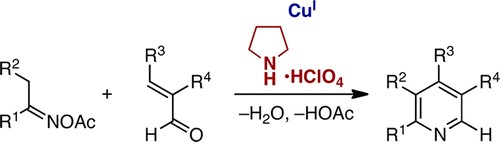
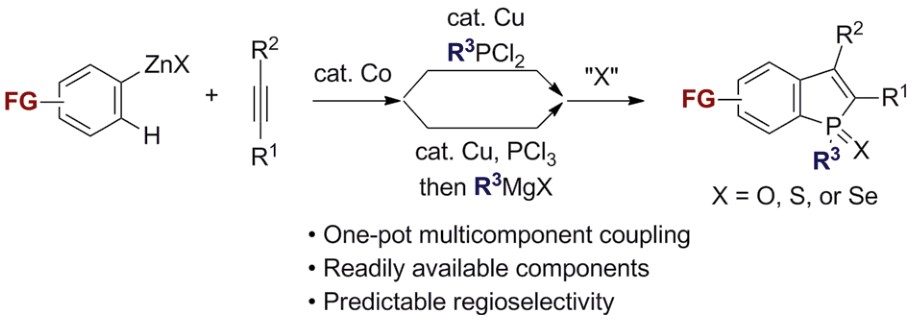
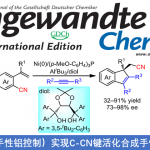
Yoshikai教授期望从大宗易得的原料出发,使用过渡金属催化合成具有骨架优势的杂环化合物。比如,他们已经实现了从N-芳基亚胺在Pd催化和空气作为氧化剂的条件下吲哚合成[8],协同的Cu /胺催化吡啶合成[9],Pd催化亚胺和炔基碘翁试剂反应合成呋喃[10],以及利用Migratory arylzincation实现苯并含磷杂环的合成[11]。
Pd-Catalyzed Aerobic Oxidative indole synthesis
Pyridine synthesis via synergistic Copper/iminium catalysis
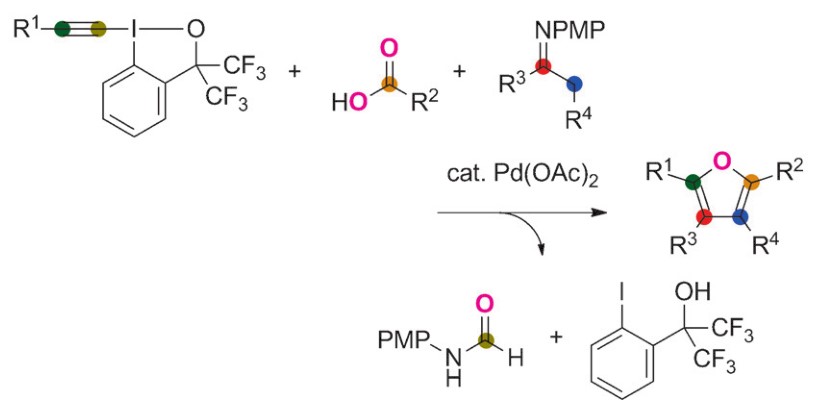
Furan synthesis via Pd catalysis of imines and alkynyliodonium reagents
Functionalized benzo[b]phosphole derivative synthesis
参考文献
- [1] For a review about cobalt-catalyzed C-H functionalization, see: Yoshikai N. Acc. Chem. Res. 2014, 47, 1208. DOI: 10.1021/ar400270x.
- [2] Book chapters about cobalt-catalyzed C-H functionalization, see: a. Cobalt-Catalyzed C–H Functionalization, Yoshikai, N. In Cobalt Catalysis in Organic Synthesis: Methods and Reactions, Wiley, 2020. DOI: 10.1002/9783527814855; b. Hydroarylation of Alkynes and Alkenes using Group 7-9 First Row Transition Metal Catalysts, Yoshikai, N. In Catalytic Hydroarylation of Carbon-Carbon Multiple Bonds, Wiley, 2018. DOI: 10.1002/9783527697649; c. Heterocycle Synthesis via Co-Catalyzed C–H Bond Functionalization, Yoshikai, N. In Transition Metal-Catalyzed Heterocycle Synthesis via C–H Activation, Wiley, 2016. DOI: 10.1002/9783527691920.
- [3] a. Yoshikai N. J. Am. Chem. Soc. 2010, 132, 12249. DOI: 10.1021/ja106814p; b. Yoshikai N. J. Am. Chem. Soc. 2011, 133, 17283. DOI: 10.1021/ja2047073; c. Yoshikai N. Angew. Chem. Int. Ed. 2012, 51, 4698. DOI: 10.1002/anie.201200019; d. Yoshikai N. Angew. Chem. Int. Ed. 2016, 55, 2870. DOI: 10.1002/anie.201510999.
- [4] a. Yoshikai N. Angew. Chem. Int. Ed. 2011, 50, 6888. DOI: 10.1002/anie.201101823; b. Yoshikai N. J. Am. Chem. Soc. 2011, 133, 400. DOI: 10.1021/ja108809u; c. Yoshikai N. Angew. Chem. Int. Ed. 2013, 52, 1240. DOI: 10.1002/anie.201207958; d. Yoshikai N. J. Am. Chem. Soc. 2014, 136, 16748. DOI: 10.1021/ja509919x; e. Yoshikai N. Angew. Chem. Int. Ed. 2014, 53, 14166. DOI: 10.1002/anie.201408028; f. Yoshikai N. Angew. Chem. Int. Ed. 2016, 55, 12731. DOI: 10.1002/anie.201605877; g. Yoshikai N. Angew. Chem. Int. Ed. 2017, 56, 2449. DOI: 10.1002/anie.201611518.
- [5] a. Yoshikai N. Chem. Commun. 2012, 48, 4305. DOI: 10.1039/C2CC31114C; b. Yoshikai N. Org. Lett. 2012, 14, 4234. DOI: 10.1021/ol301934y; c. Yoshikai N. J. Am. Chem. Soc. 2013, 135, 9279. DOI: 10.1021/ja403759x.
- [6] Yoshikai N. J. Org. Chem. 2011, 76, 1972. DOI: 10.1021/jo102417x.
- [7] a. Yoshikai N. Angew. Chem. Int. Ed. 2012, 51, 9610. DOI: 10.1002/anie.201204388; b. Yoshikai N. b. Yoshikai N. Angew. Chem. Int. Ed. 2016, 55, 336. DOI: 10.1002/anie.201508262.
- [8] Yoshikai N. J. Am. Chem. Soc. 2012, 134, 9098. DOI: 10.1021/ja3030824.
- [9] Yoshikai N. J. Am. Chem. Soc. 2013, 135, 3756. DOI: 10.1021/ja312346s.
- [10] Yoshikai N. Angew. Chem. Int. Ed. 2015, 54, 11107. DOI: 10.1002/anie.201504687.
- [11] a. Yoshikai N. Angew. Chem. Int. Ed. 2014, 53, 7543. DOI: 10.1002/anie.201404019; b. Yoshikai N. Org. Lett. 2019, 21, 3232. DOI: 10.1021/acs.orglett.9b00955.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!
























No comments yet.