本文作者:alberto-caeiro
大宮 寛久[1](Hirohisa Ohmiya),日本有机化学家,金沢大学医薬保健研究域,薬学系教授。图片:实验室介绍。
经历
- 2002 B. S., Kyoto Pharmaceutical University(京都药科大学)
- 2004 M. S., Kyoto Pharmaceutical University (Prof. 上西潤一 Jun’ichi Uenishi)
- 2007 Dr degree in Engineering, Kyoto University (京都大学)(Prof. 大嶌幸一郎 Koichiro Oshima)08
- 2007 Postdoctoral fellow, Massachusetts Institute of Technology(Prof. Timothy F. Jamison)
- 2008 Assistant Professor, Hokkaido University (北海道大学)(Prof. 澤村正也 Masaya Sawamura)
- 2010 Associate Professor, Hokkaido University (Prof. 澤村正也 Masaya Sawamura)
- 2017 Professor, Kanazawa University
- 2019 JST PRESTO Researcher
获奖经历
- 2011 The Young Scholar Lectures of The Chemical Society of Japan
- 2012 The Encouragement Award from Hokkaido Branch of the Chemical Society of Japan
- 2012 Hokkaido University President’s Award for Outstanding Research
- 2014 The Chemical Society of Japan Award for Young Chemists
- 2014 Banyu Chemist Award “BCA 2014”
- 2014 Hokkaido University Excellent Teachers
- 2015 Thieme Chemistry Journal Award 2015
- 2015 Chugai Award Synthetic Organic Chemistry, Japan
- 2015 Hokkaido University President’s Award for Outstanding Education
- 2015 Hokkaido University President’s Award for Outstanding Research
- 2015 The Young Scientists’ Prize, The Commendation for Science and Technology by MEXT
- 2018 Asian Core Program Lectureship Award(China)
- 2018 Asian Core Program Lectureship Award(Korea)
- 2019 Asian Core Program Lectureship Award(Singapore)
工作介绍
1. N–heterocyclic carbene-based (radical) organocatalysis
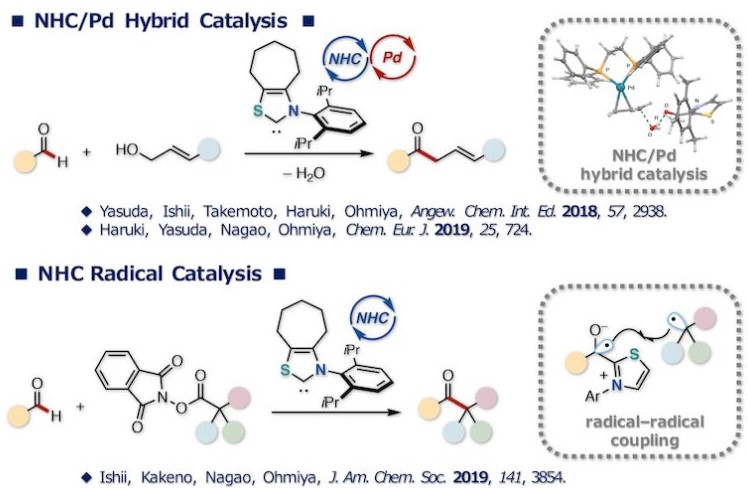
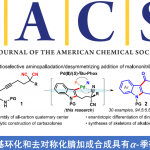
Ohmiya教授将NHC催化反应和金属催化的烯丙基取代反应结合,底物醛与小分子催化剂NHC反应生成的Breslow中间体与烯丙基金属复合物发生烯丙基取代反应,得到β,γ-不饱和酮类化合物[2]。
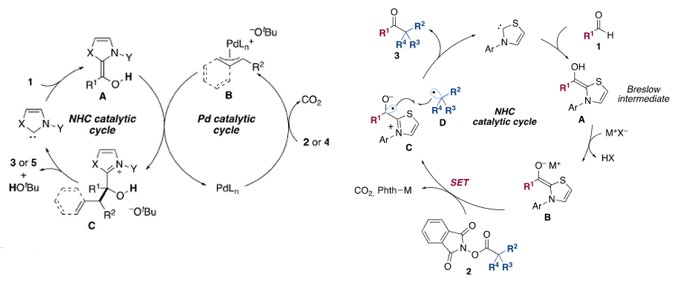
而在自由基过程中,生成的Breslow中间体去质子后,与邻苯二甲酰亚胺酯发生电子转移,生成正离子自由基与脱羧后生成的烷基自由基结合得到原料醛烷基化的酮产物[3a]。此外,该反应体系中可加入烯烃,得到插烯烷基化产物[3b]。
NHC-based (radical) organocatalysis
Reaction mechanisms: left: NHC/Pd hybrid catalysis; right: NHC radical catalysis
2. Reductive umpolung of aldehydes
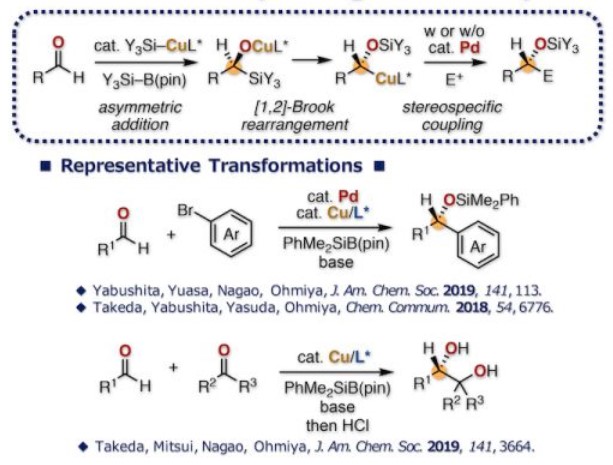
Ohmiya教授将硅基铜对醛加成反应和[1,2]-Brook重排反应结合起来,实现了醛的极性反转,形成的烷基铜物种可直接进攻酮,亚胺生成手性邻二醇[4a]和α-氨基醇[4b],也可与发生转金属,实现醛的芳基加成反应得到手性醇[4c,d]。
Reductive umpolung of aldehydes
参考文献
- [1] Ohmiya H. Angew. Chem. Int. Ed. 2018, 57, 13718. DOI: 10.1002/anie.201805108.
- [2] a. Ohmiya, H. Angew. Chem. Int. Ed. 2018, 57, 2938. DOI: 10.1002/anie.201712811; b. Ohmiya, H. Chem. Eur. J. 2019, 25, 724. DOI: 10.1002/chem.201805955; c. Ohmiya, H. Bull. Chem Soc. Jpn. 2019, 92, 937. DOI: 10.1246/bcsj.20190012.
- [3] a. Ohmiya, H. J. Am. Chem. Soc. 2019, 141, 3854. DOI: 10.1021/jacs.9b00880; b. Ohmiya, H. J. Am. Chem. Soc. 2019, 141, 14073. DOI: 10.1021/jacs.9b07194.
- [4] a. Ohmiya, H. J. Am. Chem. Soc. 2019, 141, 3664. DOI: 10.1021/jacs.8b13309; b. Ohmiya, H. Org. Lett. 2020, 22, 800. DOI: 10.1021/acs.orglett.9b04144; c. Ohmiya, H. J. Am. Chem. Soc. 2019, 141, 113. DOI: 10.1021/jacs.8b11495; d. Ohmiya, H. Chem. Commun. 2018, 54, 6776. DOI: 10.1039/c8cc01055b.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!























No comments yet.