本文作者 石油醚
概要
Nuno Maulide:(1979年12月17日出生于里斯本)葡萄牙有机化学家,奥地利维也纳大学教授。课题组主页:https://organicsynthesis.univie.ac.at/
经历
- 2003年 里斯本技术大学化学学位
- 2004年 巴黎综合理工大学和法语鲁汶大学硕士学位
- 2007年 法语鲁汶大学博士学位(Prof. István E. Markó)
- 2007年-2008年 斯坦福大学博士后(Prof. Barry M. Trost)
- 2009年-2013年 马克斯·普朗克研究所研究小组负责人
- 2013年 波鸿大学(Habilitation)
- 2013年-至今 维也纳大学有机合成教授
- 2018年-至今 CeMM的兼职PI,Organic Letters副主编,Organic Syntheses编委
获奖经历
- 2000 Merit scholarship for excellence in Chemistry studies
- 2001 Merit scholarship for excellence in Chemistry studies
- 2002 Merit scholarship for excellence in Chemistry studies
- 2002 ERASMUS scholarship for a 9-month stay at Université catholique de Louvain (Belgium)
- 2002 IAESTE scholarship for a 6-month industrial internship (Clariant AG, Basel)
- 2003 Ph.D. Scholarship from the Fonds pour la Recherche dans l’Industrie et l’Agriculture (FRIA)
- 2005 Aspirant FNRS at the Université catholique de Louvain
- 2005 Best oral communication at the YoungChem 2005
- 2005 Lhoist R&D Prize
- 2006 Best oral communication at the Frühjahrssymposium 2006
- 2007 Laureate of the DSM Awards in Science & Technology 2007
- 2007 Post-Doctoral Fellowship from the Fundação para a Ciência e Tecnologia (FCT)
- 2007 Roche Award
- 2009 Appointed Group Leader by the Max-Planck Society
- 2009 Member of the Global Young Faculty
- 2010 Thieme Journal Award
- 2011 European Research Council (ERC) Starting Grant
- 2012 ADUC Jahrespreis für Habilitanden (2011/2012)
- 2012 Bayer Early Excellence in Science Award
- 2013 Heinz Maier-Leibnitz Preis
- 2014 Wiener Mut-Preis der Wiener Vielfalt (Category: Science)
- 2015 EurJOC Young Researcher Award
- 2016 Elisabeth Lutz-Preis of the Austrian Academy of Sciences
- 2016 European Research Council (ERC) Consolidator Grant
- 2017 Aulin-Erdtman Young Investigator Lecture
- 2017 Elected to the Young Academy of the Austrian Academy of Sciences
- 2017 Förderungspreis der Stadt Wien (Incentive Award of the City of Vienna)
- 2017 Marcial Moreno Lectureship (Spanish Royal Society of Chemistry)
- 2018 Amgen/UCLA Lectureship 2018
- 2018 Elected as Corresponding Member of the Austrian Academy of Sciences
- 2018 European Research Council (ERC) Proof of Concept Grant
- 2018 Springer Heterocyclic Chemistry Award
- 2018 UNIVIE International Award
- 2019 Awarded a Christian Doppler Laboratory for Entropy-oriented drug design
- 2019 Ignaz L. Lieben–Preis in Austria
- 2019 Scientist of the Year in Austria
- 2020 Tetrahedron Young Investigator Award
研究方向
自2009年成立以来, Maulide一直致力于利用活性中间体开发反应。利用活性中间体在简单温和的反应条件下实现串联反应或不对称催化转化等多个方面研究。
1. 酰胺的活化
Maulide一直致力于利用活性中间体的反应开发,一个典型的成就是酰胺的活化。酰胺活化的途径大概分为下面两种:(1) 酰胺与三氟甲磺酸酐和碱反应,得到烯酮亚胺中间体1-2。中间体一方面进行通过分子内环化和随后的克莱森重排3,则会生成ɑ-烯丙基丁内酯。另一方面,中间体与叠氮化物4或2,6-二甲基吡啶-N-氧化物亲核加成形成新的中间体5-7,随后新的中间体通过水或者其他亲核试剂的亲核攻击形成各种用的产物。(2) 布朗斯台德酸使酰胺质子化,得到烯酮亚胺中间体8-9,烯酮亚胺中间体与金属试剂,氰化合,亚砜等化合物反应,合成ɑ,ß-二取代的烯胺10,吡啶环,异喹啉环11,嘧啶环以及酰胺的ɑ-芳基化和1,4-二羰基骨架12的构建(Fig.1)。
Figure 1 酰胺的活化
2. 过渡金属催化
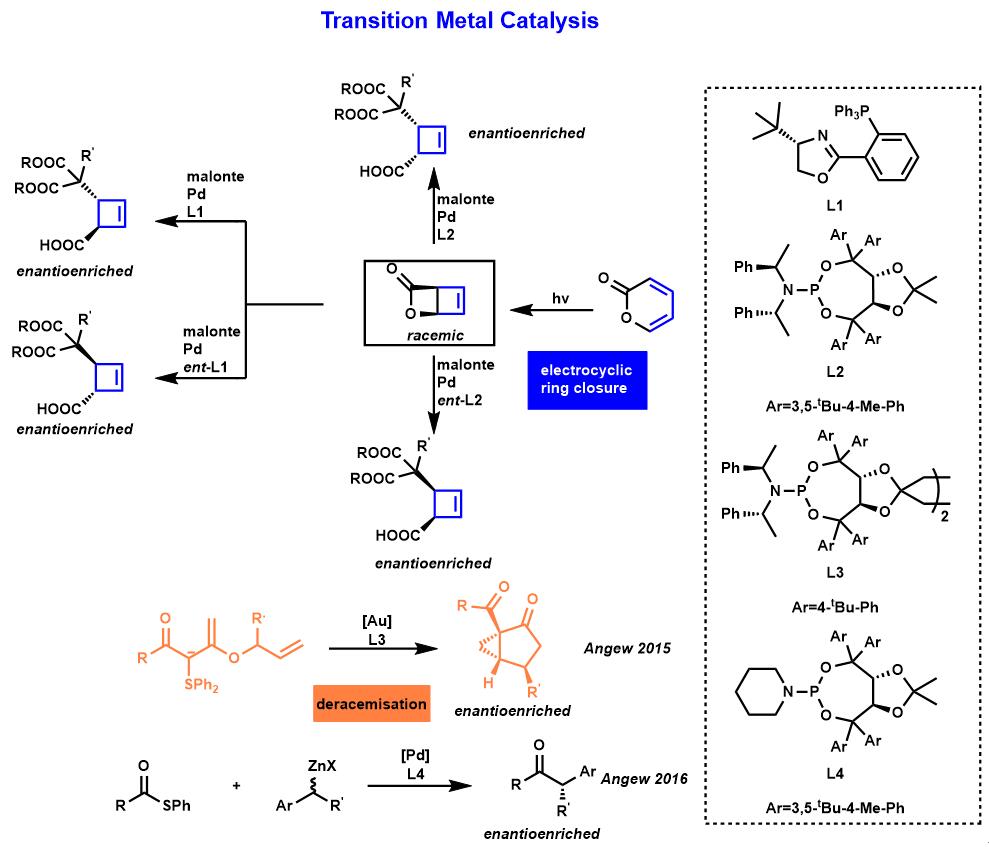
Maulide教授先前已经报道过许多过渡金属催化的反应。其中最典型的例子是高度非对映/对映选择性钯催化的环丁烯环的烯丙基烷基化13(Fig.2)。Maulide教授熟练使用手性配体成功的控制了非对映/对映选择性,高效的合成单一构型的产物14-15(Fig.2)。
Figure 2 过渡金属催化
3. 天然产物全合成
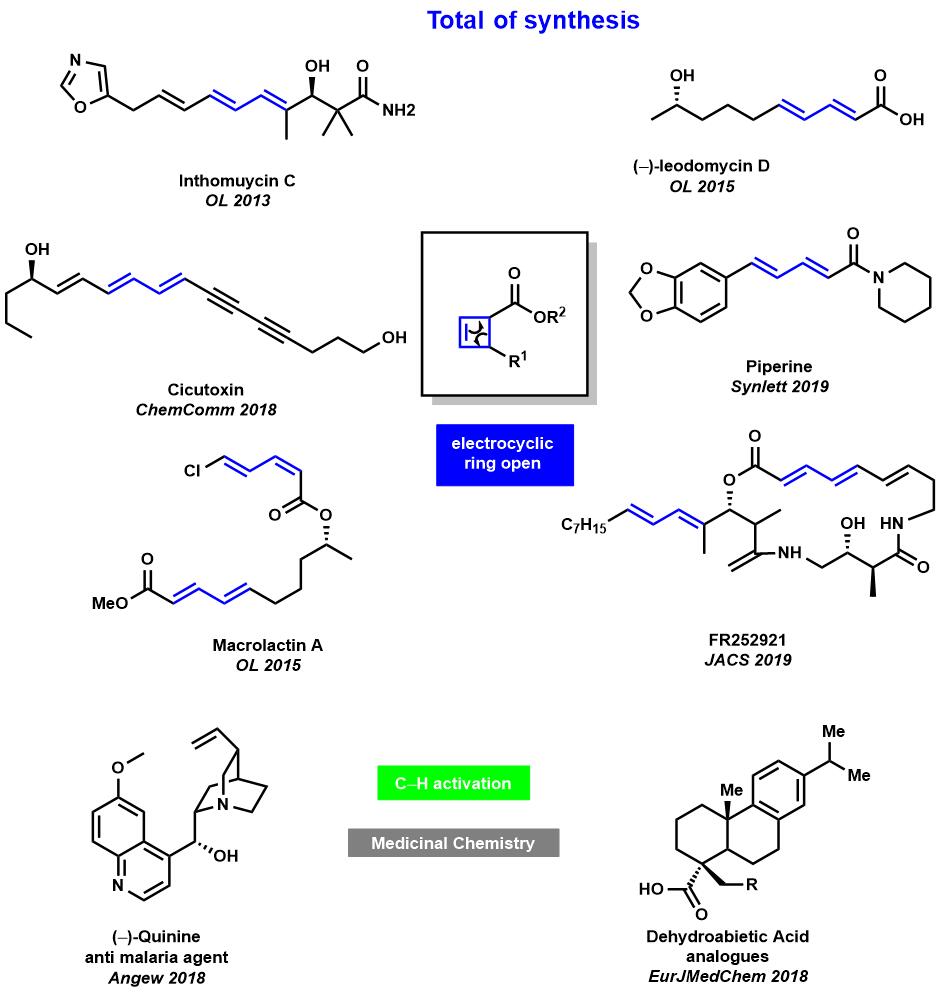
Maulide小组应用环丁烯环的4π-开环反应形成二烯骨架的方法,完成了具有共轭二烯羰基骨架的化合物的天然的全合成16-19。还使用碳氢活化20来构建具有活性的天然产物骨架(Fig.2)。
Figure 3 天然产物全合成
评论及其他
Maulide教授还精通钢琴,曾经想当钢琴家(摘自采访文章)。
Maulide教授形容自己是一名化学家和钢琴家,当他出现在电视节目中时,他通过现场钢琴表演来解释化学的研究价值。(视频1和视频2)
Maulide教授的钢琴活动仍在进行中,2012年曼彻斯特国际业余钢琴比赛的决赛入围者,以及2013年巴黎优秀业余国际钢琴比赛的第四名。 (摘自YAMAHA HP新闻与活动文章)。
Maulide教授最喜欢的词是“幸福是一种自我实现的预言。”
Maulide教授最喜欢的电影是《 Matrix》,钢琴家O Leao da Estrela。
参考文献
- [1] Ruider, S. A. & Maulide, N.Strong Bonds Made Weak: Towards the General Utility of Amides as Synthetic Modules. Angew. Chem. Int. Ed. (2015) 54, 13856-13858, doi:10.1002/anie.201508536.
- [2] de la Torre, A., Kaiser, D. & Maulide, N.Flexible and Chemoselective Oxidation of Amides to α-Keto Amides and α-Hydroxy Amides. J. Am. Chem. Soc. (2017) 139, 6578-6581, doi:10.1021/jacs.7b02983.
- [3] Madelaine, C., Valerio, V. & Maulide, N.Unexpected Electrophilic Rearrangements of Amides: A Stereoselective Entry to Challenging Substituted Lactones. Angew. Chem. Int. Ed. (2010) 49, 1583-1586, doi:10.1002/anie.200906416.
- [4] Tona, V., de la Torre, A., Padmanaban, M., Ruider, S., González, L. & Maulide, N.Chemo- and Stereoselective Transition-Metal-Free Amination of Amides with Azides. J. Am. Chem. Soc. (2016) 138, 8348-8351, doi:10.1021/jacs.6b04061.
- [5] Kaiser, D., Teskey, C. J., Adler, P. & Maulide, N.Chemoselective Intermolecular Cross-Enolate-Type Coupling of Amides. J. Am. Chem. Soc. (2017) 139, 16040-16043, doi:10.1021/jacs.7b08813.
- [6] Shaaban, S., Tona, V., Peng, B. & Maulide, N.Hydroxamic Acids as Chemoselective (ortho-Amino)arylation Reagents via Sigmatropic Rearrangement. Angew. Chem. Int. Ed. (2017) 56, 10938-10941, doi:10.1002/anie.201703667.
- [7] Adler, P., Teskey, C. J., Kaiser, D., Holy, M., Sitte, H. H. & Maulide, N.α-Fluorination of carbonyls with nucleophilic fluorine. Nat. Chem. (2019) 11, 329-334, doi:10.1038/s41557-019-0215-z.
- [8] Xie, L.-G., Shaaban, S., Chen, X. & Maulide, N.Metal-Free Synthesis of Highly Substituted Pyridines by Formal [2+2+2] Cycloaddition under Mild Conditions. Angew. Chem. Int. Ed. (2016) 55, 12864-12867, doi:10.1002/anie.201606604.
- [9] Chen, X., Ruider, S. A., Hartmann, R. W., González, L. & Maulide, N.Metal-Free meta-Selective Alkyne Oxyarylation with Pyridine N-Oxides: Rapid Assembly of Metyrapone Analogues. Angew. Chem. Int. Ed. (2016) 55, 15424-15428, doi:10.1002/anie.201607988.
- [10] Baldassari, L. L., de la Torre, A., Li, J., Lüdtke, D. S. & Maulide, N.Ynamide Preactivation Allows a Regio- and Stereoselective Synthesis of α,β-Disubstituted Enamides. Angew. Chem. Int. Ed. (2017) 56, 15723-15727, doi:10.1002/anie.201709128.
- [11] Xie, L.-G., Niyomchon, S., Mota, A. J., González, L. & Maulide, N.Metal-free intermolecular formal cycloadditions enable an orthogonal access to nitrogen heterocycles. Nat. Commun. (2016) 7, 10914, doi:10.1038/ncomms10914.
- [12] Kaldre, D., Klose, I. & Maulide, N.Stereodivergent synthesis of 1,4-dicarbonyls by traceless charge–accelerated sulfonium rearrangement. Science. (2018) 361, 664-667, doi:10.1126/science.aat5883
- [13] Luparia, M., Oliveira, M. T., Audisio, D., Frébault, F., Goddard, R. & Maulide, N.Catalytic Asymmetric Diastereodivergent Deracemization. Angew. Chem. Int. Ed. (2011) 50, 12631-12635, doi:10.1002/anie.201106321.
- [14] Klimczyk, S., Misale, A., Huang, X. & Maulide, N.Dimeric TADDOL Phosphoramidites in Asymmetric Catalysis: Domino Deracemization and Cyclopropanation of Sulfonium Ylides. Angew. Chem. Int. Ed. (2015) 54, 10365-10369, doi:10.1002/anie.201503851.
- [15] Oost, R., Misale, A. & Maulide, N.Enantioconvergent Fukuyama Cross-Coupling of Racemic Benzylic Organozinc Reagents. Angew. Chem. Int. Ed. (2016) 55, 4587-4590, doi:10.1002/anie.201600597.
- [16] Souris, C., Frébault, F., Patel, A., Audisio, D., Houk, K. N. & Maulide, N.Stereoselective Synthesis of Dienyl-Carboxylate Building Blocks: Formal Synthesis of Inthomycin C. Org. Lett. (2013) 15, 3242-3245, doi:10.1021/ol401226y.
- [17] Souris, C., Misale, A., Chen, Y., Luparia, M. & Maulide, N.From Stereodefined Cyclobutenes to Dienes: Total Syntheses of Ieodomycin D and the Southern Fragment of Macrolactin A. Org. Lett. (2015) 17, 4486-4489, doi:10.1021/acs.orglett.5b02149.
- [18] Berger, M., Chen, Y., Bampali, K., Ernst, M. & Maulide, N.Expeditious synthesis of polyacetylenic water hemlock toxins and their effects on the major GABAA receptor isoform. Chem. Commun. (2018) 54, 2008-2011, doi:10.1039/C7CC09801D.
- [19] Chen, Y., Coussanes, G., Souris, C., Aillard, P., Kaldre, D., Runggatscher, K., Maulide, N.A Domino 10-Step Total Synthesis of FR252921 and Its Analogues, Complex Macrocyclic Immunosuppressants. J. Am. Chem. Soc. (2019) 141, 13772-13777, doi:10.1021/jacs.9b07185.
- [20] O’ Donovan, D. H., Aillard, P., Berger, M., de la Torre, A., Petkova, D., Knittl-Frank, C., Maulide, N.C−H Activation Enables a Concise Total Synthesis of Quinine and Analogues with Enhanced Antimalarial Activity. Angew. Chem. Int. Ed. (2018) 57, 10737-10741, doi:10.1002/anie.201804551.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!























No comments yet.