概要
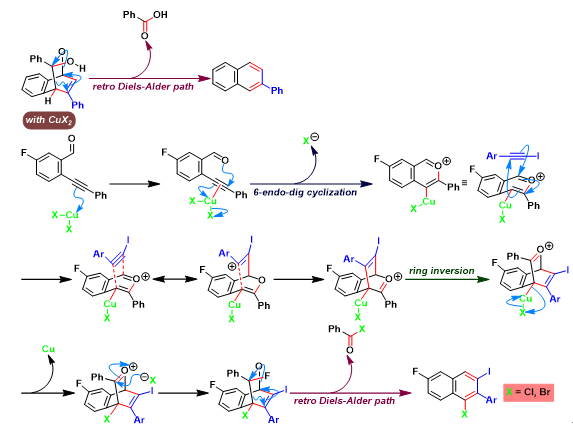
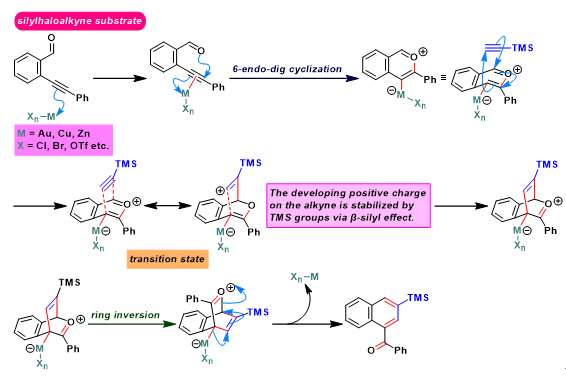
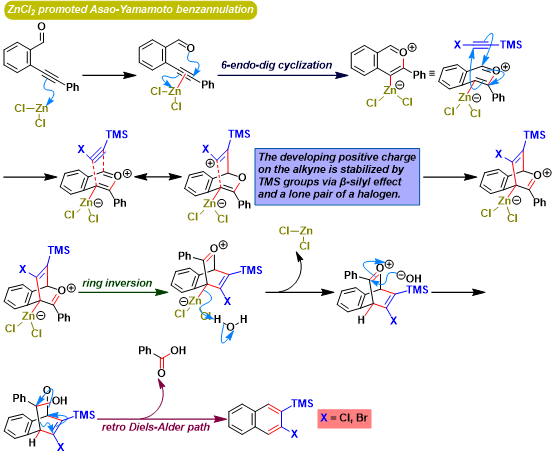
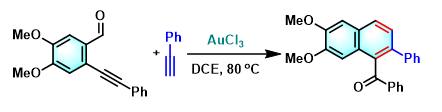
Asao-Yamamoto苯环化 (Asao-Yamamoto benzannulation)是在三卤化金[1]-[3]、AuNPore (nanoporous gold)[4]、三氟甲磺酸铜[2]-[3], [5]、卤化铜[5]或氯化锌[6]催化条件下,通过邻炔基苯甲醛或烯基炔醛 (enynal)与各类炔基化合物之间的分子间[1]-[2], [4]-[11]或分子内[3]的形式[4 + 2]苯环化过程 (formal [4 + 2] benzannulation),获得一系列萘环或苯环化合物的反应。该反应由日本Tohoku大学理学院化学系 (東北大学理学部化学教室, Department of Chemistry, Graduate School of Science, Tohoku University)的Asao (浅尾 直樹,Asao Naoki)与Yamamoto (山本 嘉則,Yamamoto Yoshinori)在2002年首次报道[1]。
Asao-Yamamoto苯环化反应具有优良的产率、温和的反应条件与优良的区域选择性。在各类萘环或苯环化合物[1]-[4], [7]-[11]、卤代与多卤代芳烃[5]-[6]、聚苯类共轭聚合物[12]的构建以及生理活性天然产物的全合成[13]中具有广阔的应用前景。
基本文献
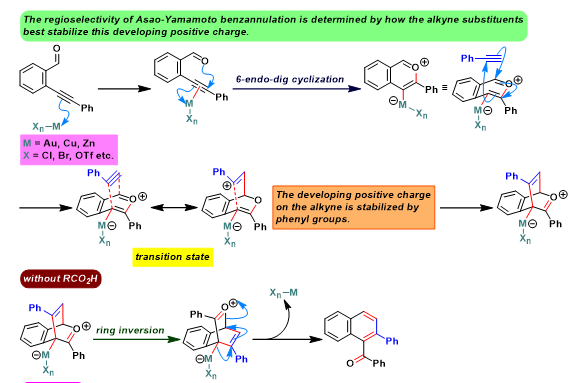
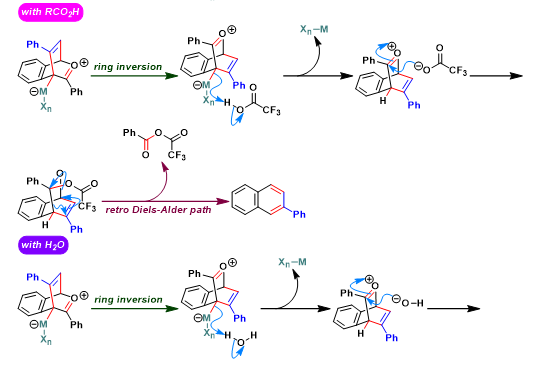
[1] N. Asao, K. Takahashi, S. Lee, T. Kasahara, Y. Yamamoto, J. Am. Chem. Soc. 2002, 124, 43, 12650. doi: 10.1021/ja028128z. [2] N. Asao, T. Nogami, S. Lee, Y. Yamamoto, J. Am. Chem. Soc. 2003, 125, 36, 10921. doi: 10.1021/ja036927r. [3] N. Asao, K. Sato, Menggenbateer, Y. Yamamoto, J. Org.Chem. 2005, 70, 3682. doi: 10.1021/jo0500434. [4] N. Asao, Y. Seya, Y. Yamamoto, M. Chen, W. Zhang, A. Inoue, Synthesis 2012, 66. doi: 10.1055/s-0031-1289527. [5] D. Lehnherr, J. M. Alzola, E. B. Lobkovsky, W. R. Dichtel, Chem. – Eur. J. 2015, 21, 18122. doi: 10.1002/chem.201503418. [6] S. J. Hein, D. Lehnherr, W. R. Dichtel, Chem. Sci. 2017, 8, 5675. doi: 10.1039/C7SC01625E. [7] H. Arslan, K. L. Walker, W. R. Dichtel, Org. Lett. 2014, 16, 5926. doi: 10.1021/ol502938y. [8] D. M. Jones, G. B. Dudley, Tetrahedron 2010, 66, 4860. doi: 10.1016/j.tet.2010.03.014. [9] S. J. Hein, H. Arslan, I. Keresztes, W. R. Dichtel, Org. Lett. 2014, 16, 4416. doi: 10.1021/ol501874s. [10] D. Lehnherr, J. M. Alzola, C. R. Mulzer, S. J. Hein, W. R. Dichtel, J. Org. Chem. 2017, 82, 2004. doi: 10.1021/acs.joc.6b02840. [11] S. J. Hein, D. Lehnherr, H. Arslan, F. J. Uribe-Romo, W. R. Dichtel, Acc. Chem. Res. 2017, 50, 11, 2776. doi: 10.1021/acs.accounts.7b00385. [12] H. Arslan, J. D. Saathoff, D. N. Bunck, P. Clancy, W. R. Dichtel, Angew. Chem. Int. Ed. 2012, 51, 12051. doi: 10.1002/anie.201206964. [13] K. Sato, N. Asao, Y. Yamamoto, J. Org. Chem. 2005, 70, 22, 8977. doi: 10.1021/jo051444m.反应机理
参考文献
[1] H. Arslan, K. L. Walker, W. R. Dichtel, Org. Lett. 2014, 16, 5926. doi: 10.1021/ol502938y. [2] S. J. Hein, D. Lehnherr, H. Arslan, F. J. Uribe-Romo, W. R. Dichtel, Acc. Chem. Res. 2017, 50, 11, 2776. doi: 10.1021/acs.accounts.7b00385. [3] N. Asao, T. Shimada, T. Shimada, Y. Yamamoto, J. Am. Chem. Soc. 2001, 123, 10899. doi: 10.1021/ja011316p. [4] N. Asao, Y. Yamamoto, Bull. Chem. Soc. Jpn. 2000, 73, 1071. doi: 10.1246/bcsj.73.1071.反应实例
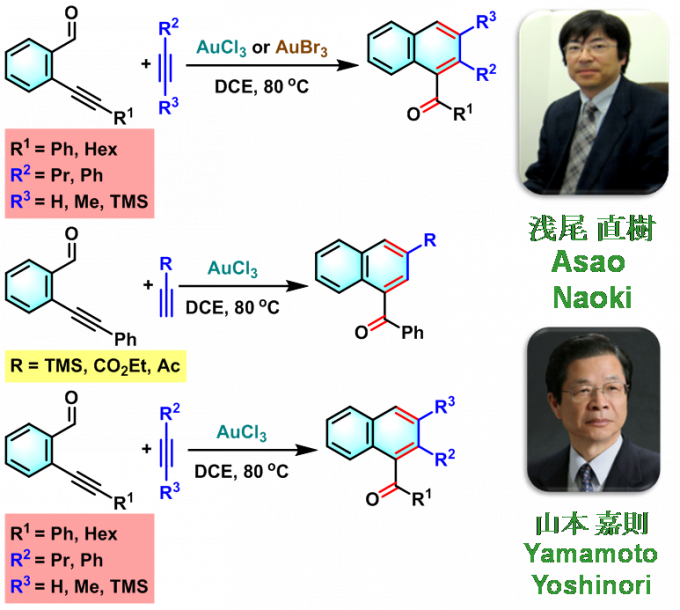
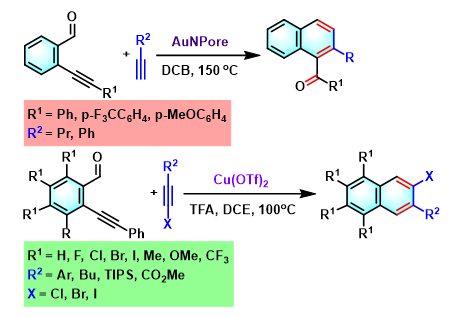
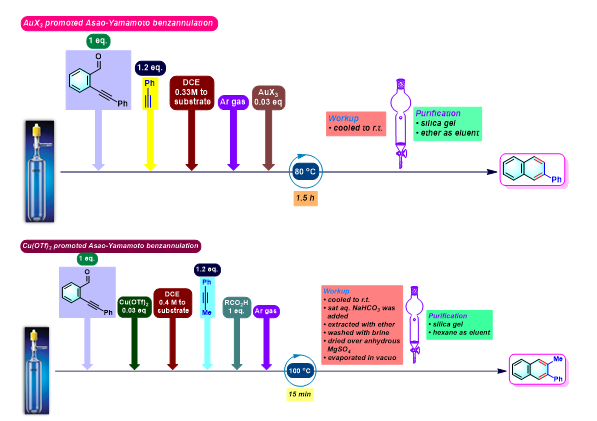
萘环化合物的合成[1]
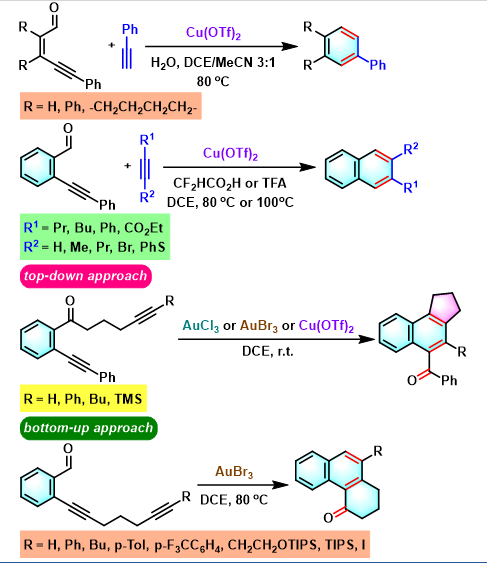
苯环化合物的合成[2]
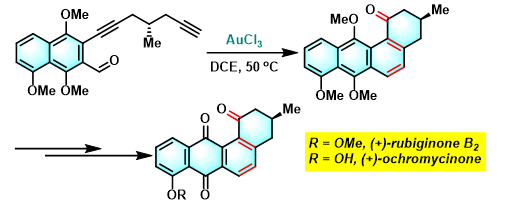
(+)-ochromycinone与(+)-rubiginoneB2的全合成[3]
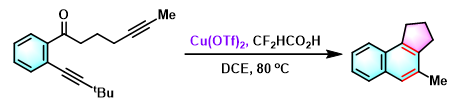
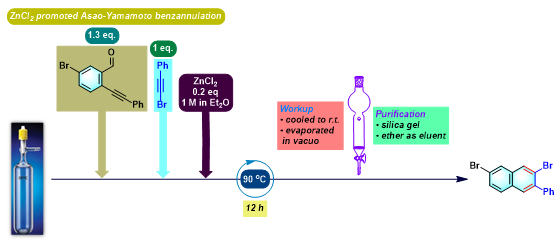
苯并茚的合成[4]
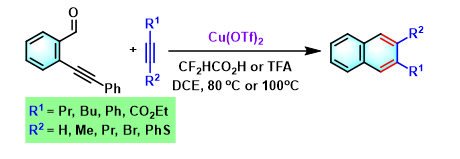
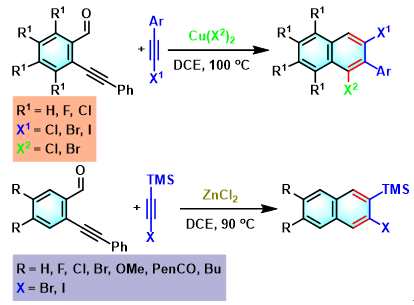
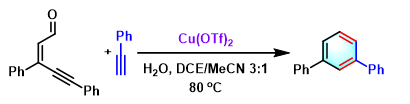
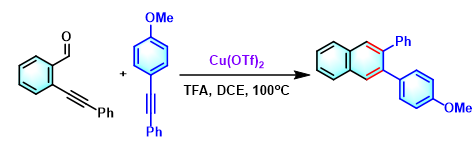
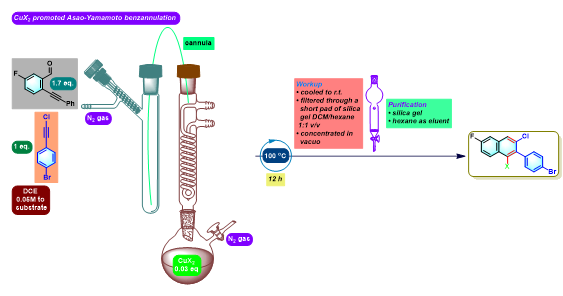
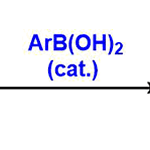
2,3-二芳基萘的合成[5]
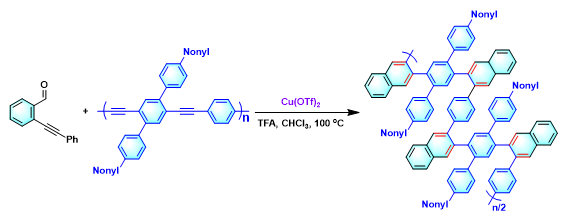
polyphenylene的合成[6]


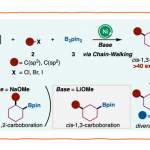
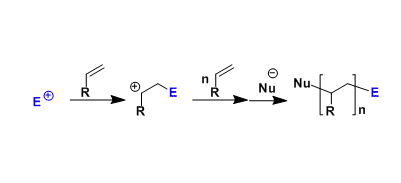
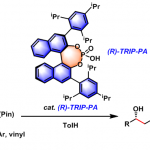











No comments yet.