概要
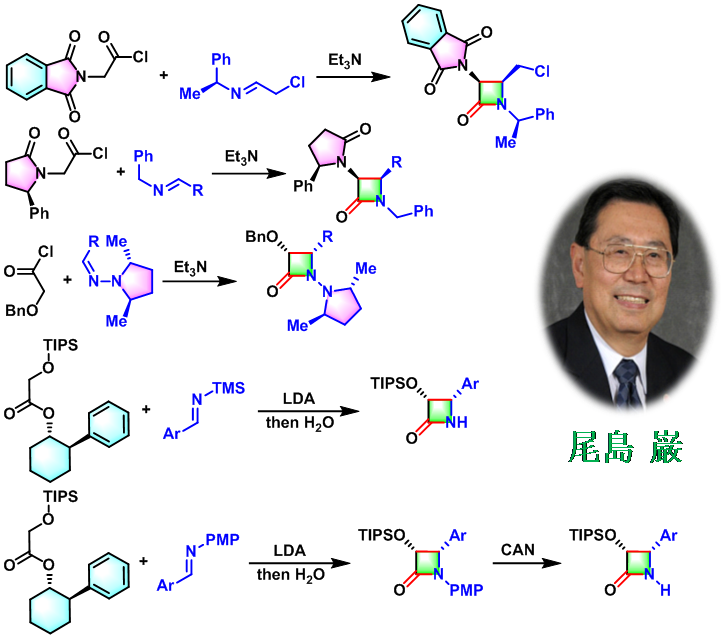
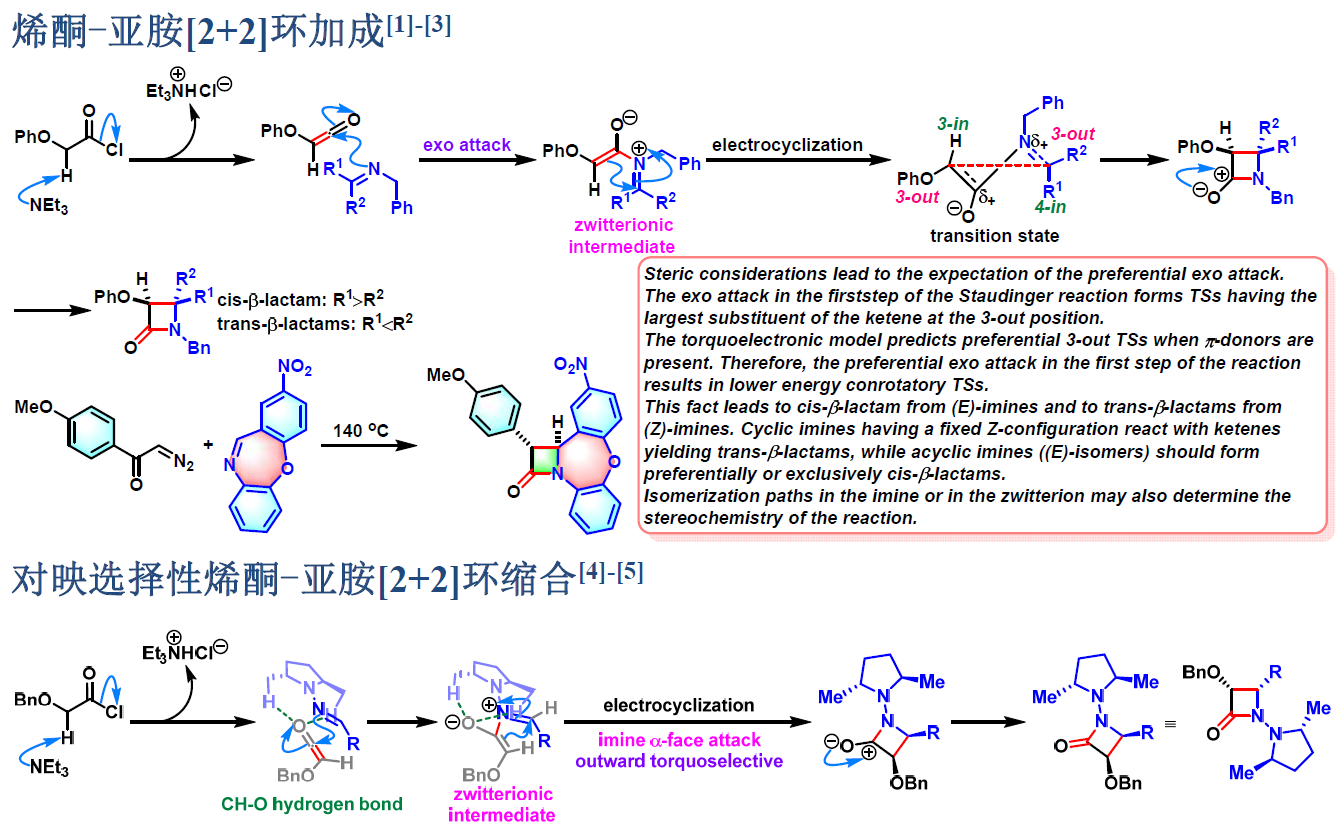
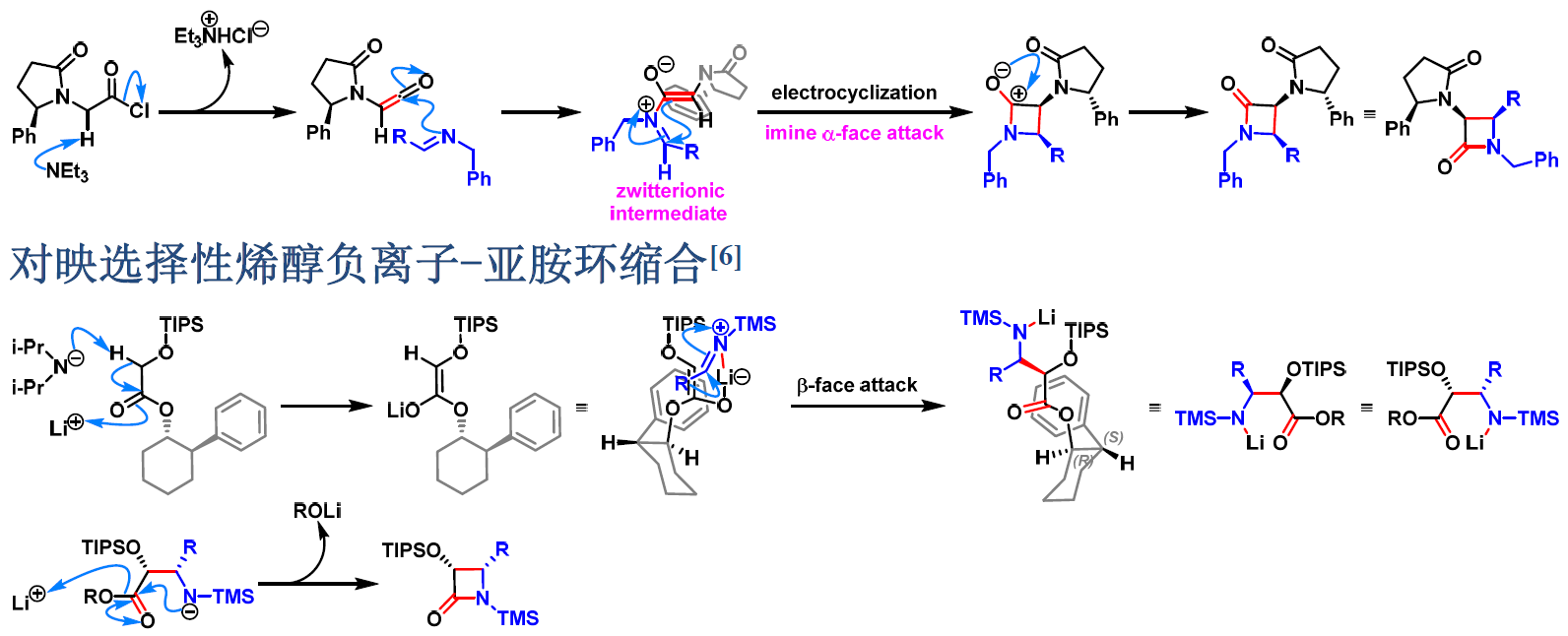
1981年开始,日本相模化学研究中心 (相模中央化学研究所, Sagami Chemical Research Center)的尾岛严 (尾島 巌,Ojima Iwao) 研究组进一步将不对称Staudinger烯酮-亚胺[2+2]环加成 (asymmetric Staudinger ketene-imine [2+2] cycloaddition) [1]-[4]及Staudinger不对称酯烯醇负离子-亚胺环缩合 (Staudinger asymmetric ester enolate-imine cyclocondensation) [5]-[7]应用于各种手性β-内酰胺类化合物的构建[1]-[7]。
同时,该课题组发现手性β-内酰胺类化合物在各类氨基酸[4], [8]-[12]、肽类[13]-[17]、非蛋白类氨基酸[18]-[19]、taxoid抗肿瘤药[20]-[25]、含氮杂环[26]-[40]及其它生物活性物质的不对称合成[41]中均可作为十分重要的合成子及合成砌块,因此,文献中将相应的手性β-内酰胺称为Ojima内酰胺 (Ojima lactam),将以手性β-内酰胺为合成子的方法称为Ojima β-内酰胺合成子方法 (Ojima β-lactam synthon method, β-LSM) [42]。
基本文献
- [1] H. Staudinger, Justus Liebigs Ann. Chem. 1907, 356, 51. doi: 10.1002/jlac.19073560106.
- [2] I. Ojima, H. J. C. Chen, J. Chem. Soc. Chem. Commun. 1987, 625. doi: 10.1039/C39870000625.
- [3] A. K. Bose, M. S. Manhas, J. C. Chib, H. P. S. Chawla, B. Dayal, J. Org. Chem. 1974, 39, 2877. doi: 10.1021/jo00933a013.
- [4] N. Hatanaka, I. Ojima, Chem. Lett. 1981, 234. doi: 10.1246/cl.1981.231.
- [5] H. Gilman, M. Speeter, J. Am. Chem. Soc. 1943, 65, 2255. doi: 10.1021/ja01251a503.
- [6] F. H. van der Steen, H. Kleijn, G. J. P. Britovsek, J. T. B. H. Jastrzebski, G.van Koten, J. Org. Chem. 1992, 57, 3906. doi: 10.1021/jo00040a034.
- [7] I. Ojima, I. Habus, Tetrahedron Lett. 1990, 31, 4289. doi: 10.1016/S0040-4039(00)97603-2.
- [8] N. Hatanaka, R. Abe, I. Ojima, Chem. Lett. 1981, 1297. doi: 10.1246/cl.1981.1297.
- [9] I. Ojima, X. Qiu, J. Am. Chem. Soc. 1987, 109, 6537. doi: 10.1021/ja00255a063.
- [10] I. Ojima, K. Nakahashi, S. M. Brandstadter, N. Hatanaka, J. Am. Chem. Soc. 1987, 109, 1798. doi: 10.1021/ja00240a033.
- [11] I. Ojima, H. J. C. Chen, K. Nakahashi, J. Am. Chem. Soc. 1988, 110, 278. doi: 10.1021/ja00209a044.
- [12] I. Ojima, Y. Pei, Tetrahedron Lett. 1990, 31, 977. doi: 10.1016/S0040-4039(00)94407-1.
- [13] I. Ojima, F. Delaloge, Chem. Soc. Rev. 1997, 26, 377. doi: 10.1039/CS9972600377.
- [14] I. Ojima, H. Wang, T. Wang, E. W. Ng, Tetrahedron Lett. 1998, 39, 923. doi: 10.1016/S0040-4039(97)10677-3.
- [15] I. Ojima, C. Sun, Y. H. Park, J. Org. Chem. 1994, 59, 1249. doi:10.1021/jo00085a008.
- [16] I. Ojima, E. W. Ng, C. Sun, Tetrahedron Lett. 1995, 36, 4547. doi: 10.1016/0040-4039(95)00825-W.
- [17] I. Ojima, T. Komata, X. Qiu, J. Am. Chem. Soc. 1990, 112, 770. doi:10.1021/ja00158a041.
- [18] I. Ojima, Y. H. Park, C. Sun, M. Zhao, T. Brigaud, Tetrahedron Lett. 1992, 33, 5737. doi: 10.1016/0040-4039(92)89019-9.
- [19] I. Ojima, H. J. C. Chen, X. Qiu, Tetrahedron 1988, 44, 5307. doi: 10.1016/S0040-4020(01)86038-5.
- [20] I. Ojima, C. Sun, M. Zucco, Y. H. Park, O. Duclos, S. Kuduk, Tetrahedron Lett. 1993, 34, 4149. doi: 10.1016/S0040-4039(00)60514-2.
- [21] I. Ojima, J. C. Slater, E. Michaud, S. D. Kuduk, P. Y. Bounaud, P. Vrignaud, M. C. Bissery, J. Veith, P. Pera, R. J. Bernacki, J. Med. Chem. 1996, 39, 3889. doi: 10.1021/jm9604080.
- [22] I. Ojima, S. D. Kuduk, J. C. Slater, R. H. Gimi, C. M. Sun, Tetrahedron, 1996, 52, 209. doi: 10.1016/0040-4020(95)00865-6.
- [23] I. Ojima, J. C. Slater, P. Pera, J. M. Veith, A. Abouabdellah, J. P. Begue, R. J. Bernacki, Bioorg. Med. Chem. Lett. 1997, 7, 133. doi: 10.1016/S0960-894X(96)00595-1.
- [24] I. Ojima, I. Habus, M. Zhao, G. I. Georg, L. R. Jayashinge, J. Org. Chem. 1991, 56, 1681. doi: 10.1021/jo00005a003.
- [25] I. Ojima, J. C. Slater, S. D. Kuduk, C. S. Takeuchi, R. H. Gimi, C. Sun, Y. H. Park, P. Pera, J. M. Veith, R. J. Bernacki, J. Med. Chem. 1997, 40, 267. doi: 10.1021/jm960563e.
- [26] K. Anushree I. Ojima, Tetrahedron 2012, 68, 10640. doi: 10.1016/j.tet.2012.07.090.
- [27] B. Alcaide, Y. Martin-Cantalejo, J. Rodriguez-Lopez, M. Sierra, J. Org. Chem. 1993, 58, 4767. doi: 10.1021/jo00069a054.
- [28] B. Alcaide, P. Almendros, G. Cabrero, M. Ruiz, Org. Lett. 2005, 7, 3981. doi: 10.1021/ol051504a.
- [29] L. A. Cabell, J. S. McMurray, Tetrahedron Lett. 2002, 43, 2491. doi: 10.1016/S0040-4039(02)00318-0.
- [30] M. Alajarin, P. Sanchez-Andrada, J. Org. Chem. 2001, 66, 8470. doi: 10.1021/jo015922e.
- [31] M. Alajarin, A. Vidal, P. Sanchez-Andrada, F. Tovar, G. Ochoa, Org. Lett. 2000, 2, 965. doi: 10.1021/ol0056168.
- [32] P. Almendros, C. Aragoncillo, G. Cabrero, R. Callejo, R. Carrascosa, A. Luna, del T. M. Campo, M. C. Pardo, M. T. Quiros, M. C. Redondo, C. Rodriguez-Ranera, A. Rodriguez-Vicente, M. P. Ruiz, Arkivoc 2010, 74. doi: 10.3998/ark.5550190.0011.308.
- [33] M. D’hooghe, S. Dekeukeleire, E. Leemans, N. De Kimpe, Pure Appl. Chem. 2010, 82, 1749. doi: 10.1351/PAC-CON-09-09-39.
- [34] N. Piens, S. De Craene, J. Franceus, K. Mollet, K. Van Hecke, T. Desmet, M. D’ hooghe, Org. Biomol. Chem. 2016, 14, 11279. doi: 10.1039/C6OB02221A.
- [35] A. Macías, E. Alonso, C. Del Pozo, J. Gonz_lez, Tetrahedron Lett. 2004, 45, 4657. doi: 10.1016/j.tetlet.2004.04.109
- [36] A. Vasudevan, C. I. Villamil, S. W. Djuric, Org. Lett. 2004, 6, 3361. doi: 10.1021/ol048638t.
- [37] B. Alcaide, P. Almendros, G. Cabrero, M. P. Ruiz, Chem. Commun. 2007, 4788. doi: 10.1039/B711473G.
- [38] P. Singh, P. Singh, K. Kumar, V. Kumar, M. P. Mahajan, K. Bisetty, Heterocycles 2012, 86, 1301. doi: 10.3987/COM-12-S(N)83.
- [39] V. Mehra, P. Singh, N. Manhas, V. Kumar, Synlettt 2014, 25, 1124. doi: 10.1055/s-0033-1341049.
- [40] K. Kumar, S. Kumar, T. Singh, A. Anand, V. Kumar, Tetrahedron Lett. 2014, 55, 3957. doi: 10.1016/j.tetlet.2014.05.016.
- [41] I. Ojima, M. Zhao, T. Yamato, K. Nakahashi, M. Yamashita, R. Abe, J. Org. Chem. 1991, 56, 5263. doi: 10.1021/jo00018a012.
- [42] I. Ojima, Acc. Chem. Res. 1995, 28, 383. doi: 10.1021/ar00057a004.
反应机理
参考文献
- [1] F. P. Cossío, A. Arrieta, M. A. Sierra, Acc. Chem. Res. 2008, 41, 925. doi: 10.1021/ar800033j.
- [2] F. P. Cossío, J. M. Ugalde, X. Lopez, B. Lecea, C. Palomo, J. Am. Chem. Soc. 1993, 115, 995. doi: 10.1021/ja00056a026.
- [3] S. Dumas, L. S. Hegedus, J. Org. Chem. 1994, 59, 4967. doi: 10.1021/jo00096a046.
- [4] E. Martín-Zamora, A. Ferrete, J. M. Llera, J. M. Munõz, R. R. Pappalardo, R. Fernández, J. M. Lassaletta, Chem. Eur. J. 2004, 10, 6111. doi: 10.1002/chem.200400452.
- [5] F. P. Cossío, A. Arrieta, B. Lecea, J. M. Ugalde, J. Am. Chem. Soc. 1994, 116, 2085. doi: 10.1021/ja00084a054.
- [6] I. Ojima, Habus, M. Zhao, M. Zucco, Y. Park, C. Sun, T. Brigaud, Tetrahedron, 1992, 48, 6985. doi: 10.1016/S0040-4020(01)91210-4.
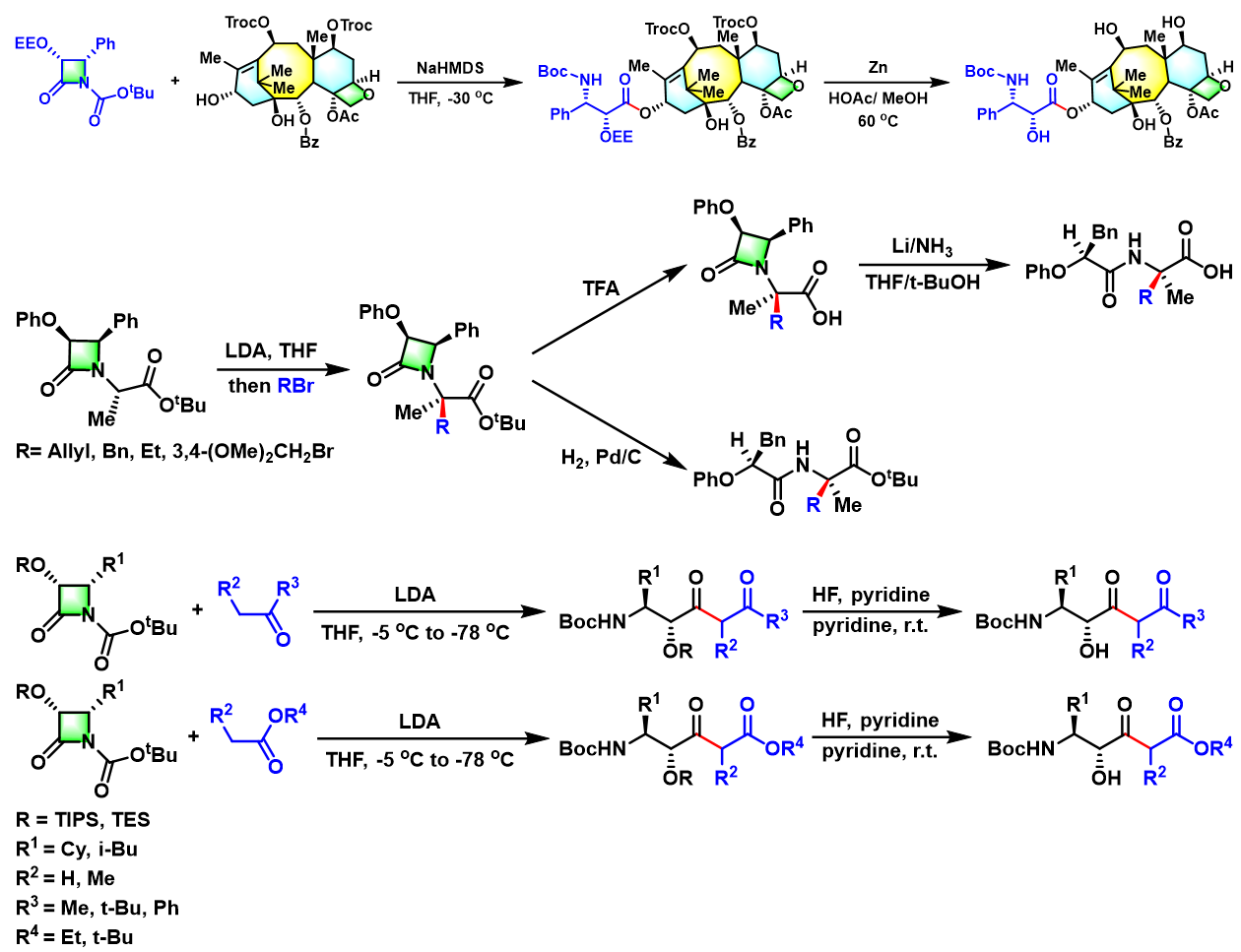
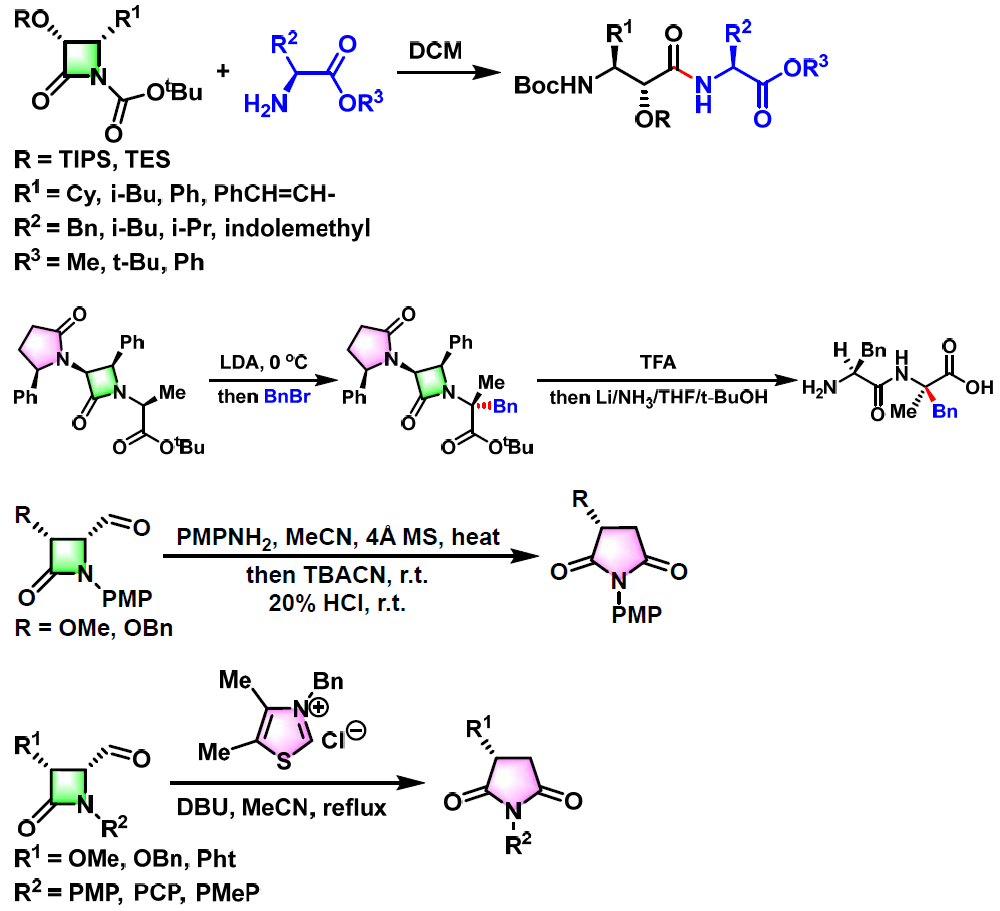
反应实例
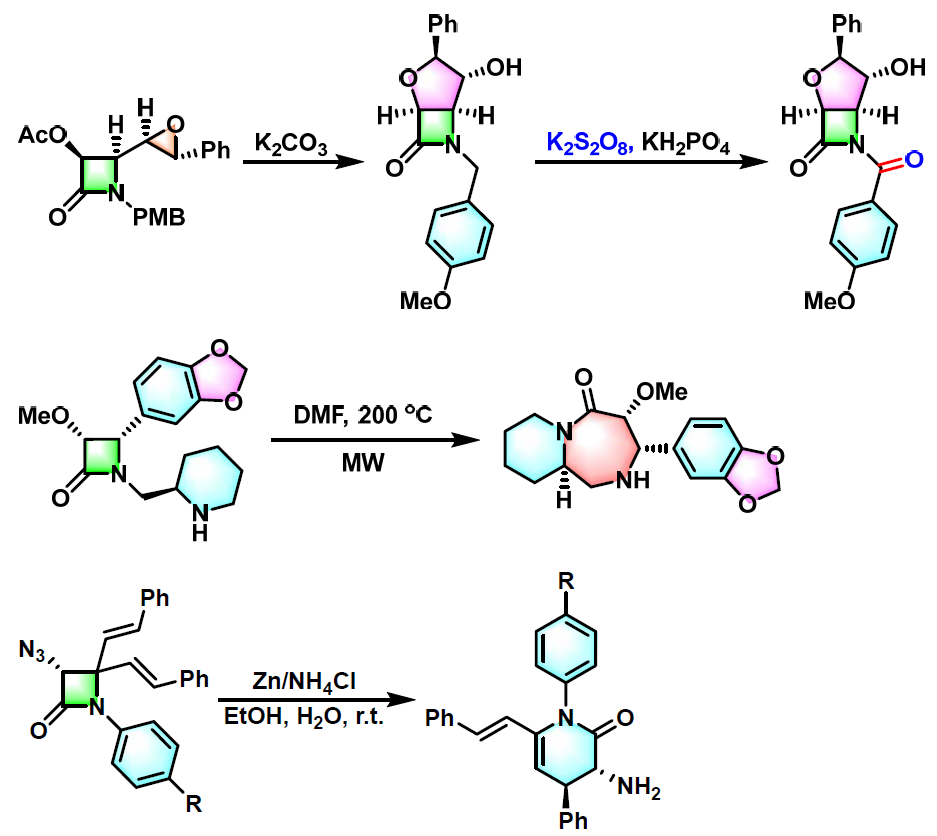
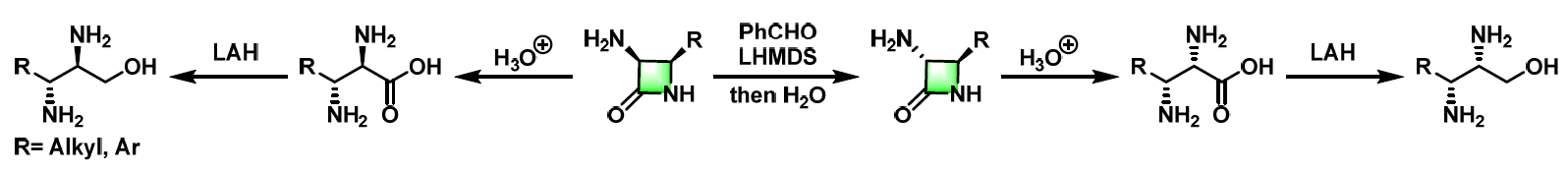
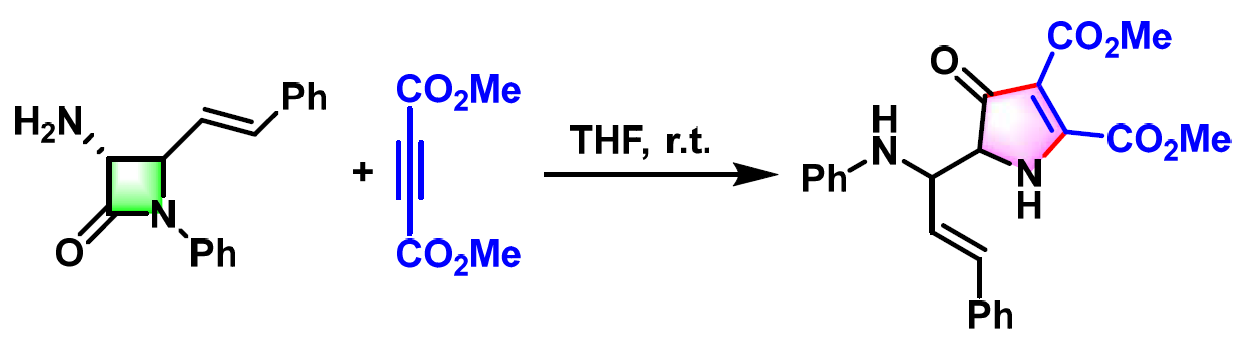
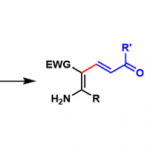
氨基酸及其衍生物的合成[1]
杂环化合物的合成[2]
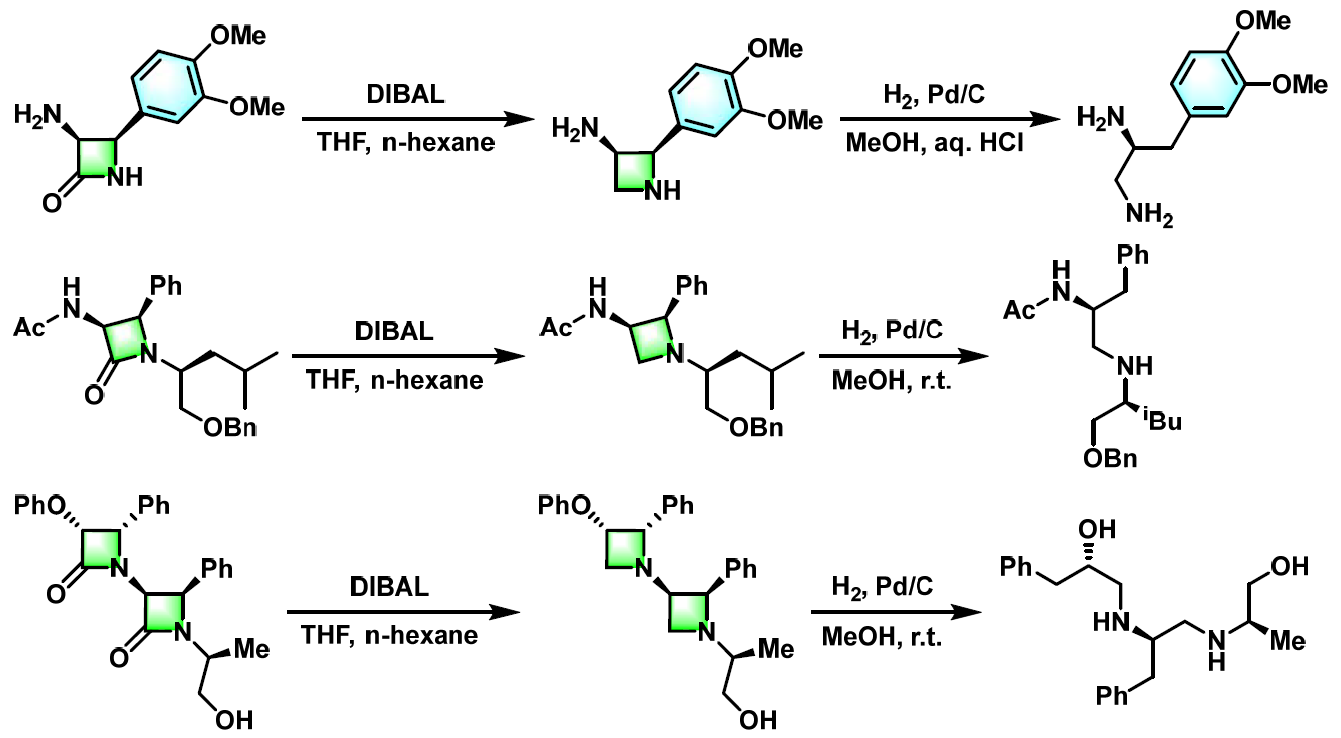
多胺、多氨基醇及醚多氨基醚的合成[3]
实验步骤
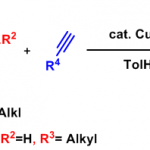
烯酮-亚胺[2+2]环加成
向圆底烧瓶中加入亚胺 (1 eq.),之后加入无水THF溶解 (维持底物浓度为0.4 M),随后,加入三乙胺 (3 eq.)。将上述混合物冷却至0 °C,随后滴加酰氯的无水THF溶液 (1.5eq., 酰氯浓度为2.5 M)。滴加结束后,升至室温,将上述反应混合物在室温下进行搅拌,直至反应结束。反应结束后,减压除去溶剂。将残余物通过二氯甲烷溶解,加入水进行洗涤,同时,继续加入二氯甲烷进行萃取。合并有机相,采用无水硫酸镁进行干燥。减压除去溶剂后,将粗产物通过快速柱色谱 (石油醚/乙酸乙酯 2:1 v/v 作为洗脱剂)分离纯化获得相应β-内酰胺产物。
烯醇负离子-亚胺环缩合
0 °C下,向二异丙胺的无水THF溶液 (浓度为0.05 M)中加入正丁基锂 (2.5 M正己烷溶液,与二异丙胺计量比为1:1)。维持0 °C,将上述均相混合物搅拌1 h,之后冷却至-78 °C。通过插管将酯底物的无水THF溶液 (1 eq., 底物浓度为0.04 M)滴加至上述原位生成的LDA (1.2 eq.)中,超过2 h后,滴加完毕。随后,将上述混合物冷却至-85 °C,再将亚胺的无水THF溶液 (1.3 eq., 底物浓度为0.05 M)滴加至上述反应体系中,超过3 h后,滴加完毕。将上述反应混合物升至室温,搅拌直至反应结束。反应结束后,加入饱和氯化铵溶液进行淬灭,淬灭完成后,加入乙醚进行萃取,将合并的有机相采用无水硫酸镁进行干燥,随后,减压除去溶剂。将粗产物通过硅胶柱色谱 (正己烷/乙酸乙酯 5:1 v/v 作为洗脱剂)分离纯化获得相应β-内酰胺产物。
参考文献
- [1] I. Ojima, Acc. Chem. Res. 1995, 28, 383. doi: 10.1021/ar00057a004.
- [2] P. Sharma, M. J. K. Mann, B. Kuila, P. Singh, G. Bhargava, Synlett 2016, 27, 422. doi: 10.1055/s-0035-1560826.
- [3] I. Ojima, M. Zhao, T. Yamato, K. Nakahashi, M. Yamashita, R. Abe, J. Org. Chem 1991, 56, 5263. doi: 10.1021/jo00018a012.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!

















No comments yet.