本文作者:石油醚
概要
M. Kevin Brown,印第安纳大学化学系副教授,有机化学家。课题组主页:https://kbrown.lab.indiana.edu/
经历
- 1998-2002年 汉密尔顿学院化学学士(Professor Ian J. Rosenstein)
- 2002-2008年 波士顿学院有机化学博士(Professor Amir H. Hoveyda)
- 2008-2011年 哈佛大学博士后(Professor E. J. Corey)
- 2011-2017年 印第安纳大学化学系助理教授
- 2017- 印第安纳大学化学系副教授
获奖经历
- 2020 Humboldt Fellowship for Experienced Researchers
- 2019 Outstanding Reviewer, Chemical Science
- 2019 NIH MIRA for Established Investigators
- 2016 Novartis Early Career Award
- 2016 Amgen Young Investigator Award
- 2016 National Science Foundation CAREER Award, 2016
- 2015 Sloan Research Fellowship, 2015
- 2014 IU Trustees Teaching Award, 2014
- 2013 Thieme Chemistry Journal Awardee, 2013
- 2008-2011 National Institutes of Health, Ruth L. Kirschstein National Research Service Award,
- Harvard University, 2008-2011
- 2010 ESF Research Conference on Natural Products Chemistry, Biology and Medicine III,
- Travel Award. European Science Foundation, 2010
- 2007 Bristol-Myers Squibb Graduate Fellowship in Synthetic Organic Chemistry, Sponsored by
- Bristol-Myers Squibb
- 2006 Graduate School of Arts and Sciences Academic Achievement Award Boston College
- 2006 Excellence in Chemistry Award, Roche Biosciences
- 2005 Graduate Fellowship in Organic Chemistry, American Chemical Society, Sponsored by
- Schering-Plough
- 2002 Underwood Prize in Chemistry, Hamilton College
- 2002 Elihu Root Fellowship, Hamilton College
- 2002 Sigma Xi Scientific Research Society, Hamilton College
研究方向
Brown教授小组致力于有机合成方法学研究以及合成新策略的开发。其小组对以下三个研究领域广泛感兴趣:1)立体选择性[2+2]环加成反应制备环丁烷;2)铜催化的交叉偶联/间断的交叉偶联反应;3)钯/铜协同催化Csp3-亲核试剂立体选择性交叉偶联。
1. 立体选择性[2 + 2]环加成1-5
环丁烷的骨架广泛存在于天然产物分子中。Brown教授小组致力于开发催化剂控制烯烃与丙二烯或杂丙二烯的立体选择性[2 + 2]环加成反应。Brown教授小组开发的立体选择性[2 + 2]环加成反应要么直接用于生物活性分子构建6-8,要么间接用于快速构建多种复杂性分子的多环框架6,9-11(图1)。
图 1 立体选择性[2 + 2]环加成
2. 过渡金属催化的交叉偶联反应
过渡金属催化的交叉偶联反应在重要分子化学合成中的价值不可低估。在这一领域,Brown的实验室主要关注两个方面:1) 铜催化的铃木型交叉偶联/间断交叉偶联;2) Csp3-亲核试剂的立体选择性交叉偶联反应。
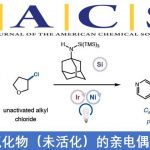
(1) Csp3-亲核试剂的立体选择性交叉偶联12-21
传统的Csp3-亲核试剂的立体选择性交叉偶联的策略包括手性亲核试剂预合成和分离亲核试剂。Brown教授设想了一种不同以往的方法来解决这个问题,即通过在简单的烯烃上加入Ln[M]-G (Bpin, H, Ar等)配合物,然后通过交叉偶联原位催化生成Csp3-有机金属亲核试剂。该方法具有几个显着的优点:1)简单的烯烃用作化学原料; 2)烯烃的两个位置均可官能化,因此允许在一个步骤中生成两个新键(最多两个立体中心); 3)可以合并一个用于进一步官能团化的策略;4)Csp3-亲核试剂不需要预生成和分离(图 2)。
图 2 Csp3-亲核试剂的立体选择性交叉偶联
(2) 铜催化的Suzuki-Miyaura型交叉偶联22-25
Brown教授的实验室对开发实用的Cu催化Suzuki-Miyaura型交叉偶联反应感兴趣。在这一领域的努力不仅是由铜的成本和毒性带来的收益驱动,而且还得益于新反应性的发现,该反应性将使以前难以获得的重要分子得以合成。该领域的重点是利用铜的独特反应性,通过间断的交叉偶联实现烯烃官能化(图 3)。
图 3 铜催化的Suzuki-Miyaura型交叉偶联
参考文献
- [1] Ni, D. et al. Stereoselective [4+2]-Cycloaddition with Chiral Alkenylboranes. Angew. Chem. Int. Ed. (2020). 59, 11432-11439, doi:10.1002/anie.202000652.
- [2] Wiest, J. M., Conner, M. L. & Brown, M. K. Allenoates in Enantioselective [2+2] Cycloadditions: From a Mechanistic Curiosity to a Stereospecific Transformation. J. Am. Chem. Soc. (2018). 140, 15943-15949, doi:10.1021/jacs.8b10008.
- [3] Wiest, J. M., Conner, M. L. & Brown, M. K. Synthesis of (−)-Hebelophyllene E: An Entry to Geminal Dimethyl-Cyclobutanes by [2+2] Cycloaddition of Alkenes and Allenoates. Angew. Chem. Int. Ed. (2018). 57, 4647-4651, doi:10.1002/anie.201801110.
- [4] Line, N. J., Witherspoon, B. P., Hancock, E. N. & Brown, M. K. Synthesis of ent-[3]-Ladderanol: Development and Application of Intramolecular Chirality Transfer [2+2] Cycloadditions of Allenic Ketones and Alkenes. J. Am. Chem. Soc. (2017). 139, 14392-14395, doi:10.1021/jacs.7b09844.
- [5] Rasik, C. M. & Brown, M. K. Lewis Acid-Promoted Ketene–Alkene [2 + 2] Cycloadditions. J. Am. Chem. Soc. (2013). 135, 1673-1676, doi:10.1021/ja3103007.
- [6] Hancock, E. N., Kuker, E. L., Tantillo, D. J. & Brown, M. K. Lessons in Strain and Stability: Enantioselective Synthesis of (+)-[5]-Ladderanoic Acid. Angew. Chem. Int. Ed. (2020). 59, 436-441, doi:10.1002/anie.201910901.
- [7] Xu, Y., Conner, M. L. & Brown, M. K. Cyclobutane and Cyclobutene Synthesis: Catalytic Enantioselective [2+2] Cycloadditions. Angew. Chem. Int. Ed. (2015). 54, 11918-11928, doi:10.1002/anie.201502815.
- [8] Rasik, C. M. & Brown, M. K. Total Synthesis of Gracilioether F: Development and Application of Lewis Acid Promoted Ketene–Alkene [2+2] Cycloadditions and Late-Stage CH Oxidation. Angew. Chem. Int. Ed. (2014). 53, 14522-14526, doi:10.1002/anie.201408055.
- [9] Guo, R., Witherspoon, B. P. & Brown, M. K. Evolution of a Strategy for the Enantioselective Synthesis of (−)-Cajanusine. J. Am. Chem. Soc. (2020). 142, 5002-5006, doi:10.1021/jacs.0c00359.
- [10] Hancock, Erin N., Wiest, J. M. & Brown, M. K. Recent advances in the synthesis of gem-dimethylcyclobutane natural products. Nat. Prod. Rep. (2019). 36, 1383-1393, doi:10.1039/C8NP00083B.
- [11] McCallum, M. E., Rasik, C. M., Wood, J. L. & Brown, M. K. Collaborative Total Synthesis: Routes to (±)-Hippolachnin A Enabled by Quadricyclane Cycloaddition and Late-Stage C–H Oxidation. J. Am. Chem. Soc. (2016). 138, 2437-2442, doi:10.1021/jacs.5b13586.
- [12] Huang, Y. & Brown, M. K. Synthesis of Bisheteroarylalkanes by Heteroarylboration: Development and Application of a Pyridylidene–Copper Complex. Angew. Chem. Int. Ed. (2019). 58, 6048-6052, doi:10.1002/anie.201902238.
- [13] Bergmann, A. M., Dorn, S. K., Smith, K. B., Logan, K. M. & Brown, M. K. Catalyst-Controlled 1,2- and 1,1-Arylboration of α-Alkyl Alkenyl Arenes. Angew. Chem. Int. Ed. (2019). 58, 1719-1723, doi:10.1002/anie.201812533.
- [14] Gao, P., Chen, L.-A. & Brown, M. K. Nickel-Catalyzed Stereoselective Diarylation of Alkenylarenes. J. Am. Chem. Soc. (2018). 140, 10653-10657, doi:10.1021/jacs.8b05680.
- [15] Logan, K. M., Sardini, S. R., White, S. D. & Brown, M. K. Nickel-Catalyzed Stereoselective Arylboration of Unactivated Alkenes. J. Am. Chem. Soc. (2018). 140, 159-162, doi:10.1021/jacs.7b12160.
- [16] Huang, Y., Smith, K. B. & Brown, M. K. Copper-Catalyzed Borylacylation of Activated Alkenes with Acid Chlorides. Angew. Chem. Int. Ed. (2017). 56, 13314-13318, doi:10.1002/anie.201707323.
- [17] Sardini, S. R. & Brown, M. K. Catalyst Controlled Regiodivergent Arylboration of Dienes. J. Am. Chem. Soc. (2017). 139, 9823-9826, doi:10.1021/jacs.7b05477.
- [18] Smith, K. B. & Brown, M. K. Regioselective Arylboration of Isoprene and Its Derivatives by Pd/Cu Cooperative Catalysis. J. Am. Chem. Soc. (2017). 139, 7721-7724, doi:10.1021/jacs.7b04024.
- [19] Logan, K. M. & Brown, M. K. Catalytic Enantioselective Arylboration of Alkenylarenes. Angew. Chem. Int. Ed. (2017). 56, 851-855, doi:10.1002/anie.201609844.
- [20] Logan, K. M., Smith, K. B. & Brown, M. K. Copper/Palladium Synergistic Catalysis for the syn- and anti-Selective Carboboration of Alkenes. Angew. Chem. Int. Ed. (2015). 54, 5228-5231, doi:10.1002/anie.201500396.
- [21] Sardini, S. R. et al. Ni-Catalyzed Arylboration of Unactivated Alkenes: Scope and Mechanistic Studies. J. Am. Chem. Soc. (2019). 141, 9391-9400, doi:10.1021/jacs.9b03991.
- [22] Smith, K. B., Huang, Y. & Brown, M. K. Copper-Catalyzed Heteroarylboration of 1,3-Dienes with 3-Bromopyridines: A cine Substitution. Angew. Chem. Int. Ed. (2018). 57, 6146-6149, doi:10.1002/anie.201801139.
- [23] You, W. & Brown, M. K. Catalytic Enantioselective Diarylation of Alkenes. J. Am. Chem. Soc. (2015). 137, 14578-14581, doi:10.1021/jacs.5b10176.
- [24] You, W. & Brown, M. K. Diarylation of Alkenes by a Cu-Catalyzed Migratory Insertion/Cross-Coupling Cascade. J. Am. Chem. Soc. (2014). 136, 14730-14733, doi:10.1021/ja509056j.
- [25] Zhou, Y., You, W., Smith, K. B. & Brown, M. K. Copper-Catalyzed Cross-Coupling of Boronic Esters with Aryl Iodides and Application to the Carboboration of Alkynes and Allenes. Angew. Chem. Int. Ed. (2014). 53, 3475-3479, doi:10.1002/anie.201310275.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!

























No comments yet.