本文作者:石油醚
概要
Corey R. J. Stephenson(出生于加拿大),美国密歇根大学化学系教授。有机化学家课题组主页:https://www.thestephensongroup.org/
经历
- 1998年 滑铁卢大学获得学士学位
- 2005年 匹兹堡大学获得博士学位(Professor Peter Wipf)
- 1998.9-2005.2 匹兹堡大学研究生助理(Professor Peter Wipf)
- 2005.3-2007.8 苏黎世联邦理工学院博士后研究员 (Professor Erick M. Carreira)
- 2007.9-2013.2 波士顿大学化学系助理教授
- 2013.2-2013.6 波士顿大学化学系副教授
- 2014.12 明斯特大学客座教授
- 2013.7-2015.8 密歇根大学化学系副教授
- 2016.6 以色列理工学院客座教授
- 2015.9- 密歇根大学化学系副教授
获奖经历
- Thomson-Reuters Highly Cited Researcher, 2015, 2016
- Pfizer Green Chemistry Award, 2015
- EROS Best Reagent Award, 2014
- Camille Dreyfus Teacher-Scholar Award, 2013–2018
- Eli Lilly Grantee Award, 2013–2015
- Novartis Early Career Award in Organic Chemistry, 2012–2015
- Amgen Young Investigator Award, 2011
- Alfred P. Sloan Research Fellow, 2011–2013
- NSF Career Award, 2011–2016
- Boehringer–Ingelheim New Investigator Award, 2010
- Thieme Synthesis/Synlett Journal Award, 2009
- ACS Petroleum Research Foundation Type G Award, 2008
研究方向
1. 光氧化还原技术
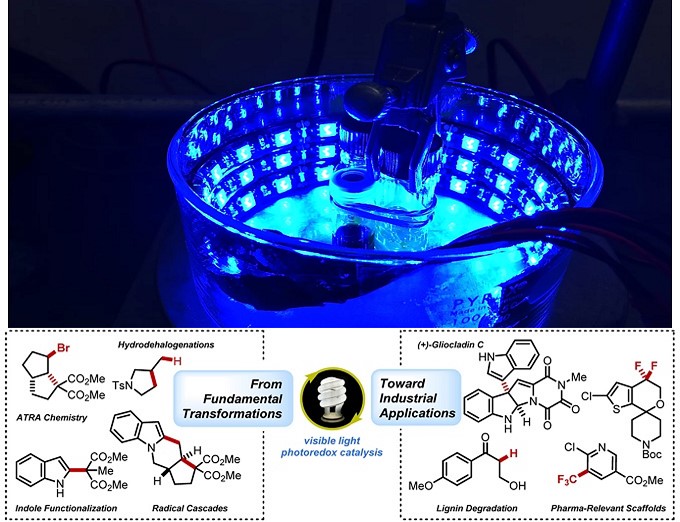
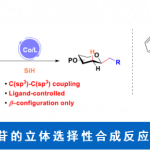
光敏电子转移催化剂为自由基中间体提供了独特的化学合成途径、拓宽了自由基中间体的应用范围以及为小分子催化领域提供了新的合成方法。该方法的优势在于温和的反应条件下,具有敏感官能团的化合物能发生化学选择性反应得到新化学分子。Stephenson课题组创造性地设计和应用自由基反应1-9促进不可持续反应的发生,从而推动光氧化还原催化领域的发展(图 1)。
图 1 光氧化还原技术
2. 复杂分子的合成
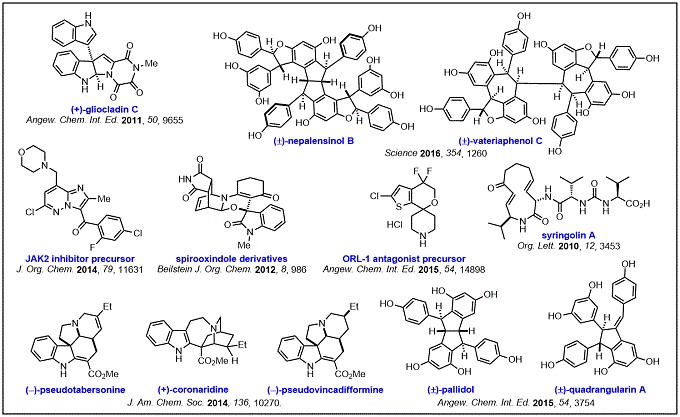
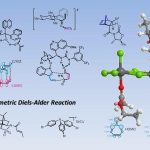
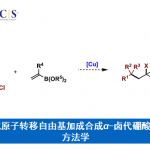
Stephenson课题组的研究重点是创造性的实现自由基中间体向复杂的生物活性分子的转化,同时致力于有机化学可持续性和可扩展性。目前,Stephenson课题组致力于应用光氧化还原催化,电化学和仿生持久性产生自由基前体10的策略来获得天然产物和与临床相关的骨架,且已经取得了不错的进展11-18。这些方法在战略层面上的优势以及课题组与行业的牢固联系为大规模合成天然产物提供了独特的机会19(图 2)。
图 2 复杂分子的合成
3. 生物质降解
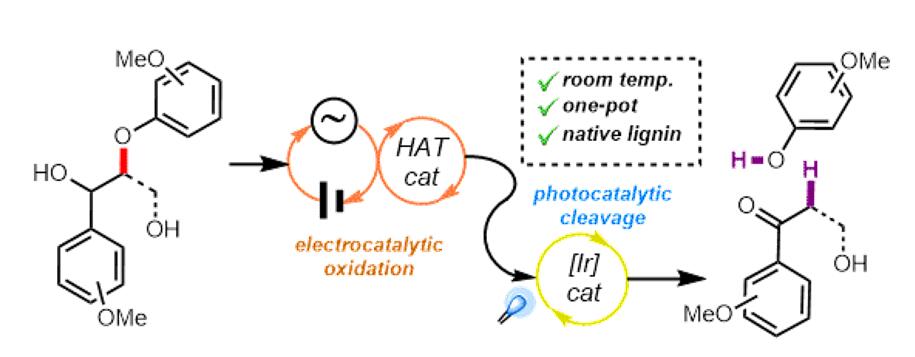
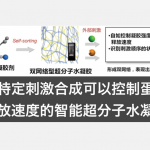
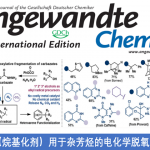
生物质(尤其是木质素)可用于合成有用的商业化学品以及燃料,具有重要的合成价值。Stephenson课题组致力于应用光和电化学的氧化还原催化来开发实用和可扩展的技术20并在温和有效的条件下促进生物质降解,为我们的美好生活提供有用的商业化学品。同时,Stephenson教授相信生物质降解领域的不断发展将为获取碳原料提供一种新的途径,从而降低化石燃料对环境的影响(图 3)。
图 3 生物质降解
4. 流态合成
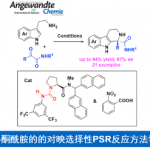
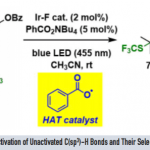
Stephenson教授对流态化学在光氧化还原催化中的应用感兴趣。课题组的目标是使用连续流态技术:(i)通过提高光透过率来有效扩大与工业相关的光氧化还原反应得应用21(ii)设计用于药物和生物质转化应用的连续多步反应22,以及(iii)开发微流体自动优化光氧化还原反应的技术(图 4)。
图 4 流态合成
参考文献
- [1] McAtee, R. C., Noten, E. A. & Stephenson, C. R. J. Arene dearomatization through a catalytic N-centered radical cascade reaction. Nat. Commun. (2020). 11, 2528, doi:10.1038/s41467-020-16369-4.
- [2] Staveness, D., Collins Iii, J. L., McAtee, R. C. & Stephenson, C. R. J. Exploiting Imine Photochemistry for Masked N-Centered Radical Reactivity. Angew. Chem. Int. Ed. (2019). 58, 19000-19006, doi:10.1002/anie.201909492.
- [3] Magallanes, G. et al. Selective C–O Bond Cleavage of Lignin Systems and Polymers Enabled by Sequential Palladium-Catalyzed Aerobic Oxidation and Visible-Light Photoredox Catalysis. ACS Catal. (2019). 9, 2252-2260, doi:10.1021/acscatal.8b04172.
- [4] Staveness, D. et al. Providing a New Aniline Bioisostere through the Photochemical Production of 1-Aminonorbornanes. Chem (2019). 5, 215-226, doi:10.1016/j.chempr.2018.10.017.
- [5] Alpers, D., Cole, K. P. & Stephenson, C. R. J. Visible Light Mediated Aryl Migration by Homolytic C−N Cleavage of Aryl Amines. Angew. Chem. Int. Ed. (2018). 57, 12167-12170, doi:10.1002/anie.201806659.
- [6] Monos, T. M., McAtee, R. C. & Stephenson, C. R. J. Arylsulfonylacetamides as bifunctional reagents for alkene aminoarylation. Science. (2018). 361, 1369-1373, doi:10.1126/science.aat2117.
- [7] Staveness, D., Bosque, I. & Stephenson, C. R. J. Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer. Acc. Chem. Res. (2016). 49, 2295-2306, doi:10.1021/acs.accounts.6b00270.
- [8] Beatty, J. W., Douglas, J. J., Cole, K. P. & Stephenson, C. R. J. A scalable and operationally simple radical trifluoromethylation. Nat. Commun. (2015). 6, 7919, doi:10.1038/ncomms8919.
- [9] Nguyen, J. D., D’Amato, E. M., Narayanam, J. M. R. & Stephenson, C. R. J. Engaging unactivated alkyl, alkenyl and aryl iodides in visible-light-mediated free radical reactions. Nat. Chem. (2012). 4, 854-859, doi:10.1038/nchem.1452.
- [10] Romero, K. J., Galliher, M. S., Pratt, D. A. & Stephenson, C. R. J. Radicals in natural product synthesis. Chemical Society Reviews (2018). 47, 7851-7866, doi:10.1039/C8CS00379C.
- [11] Romero, K. J. et al. Synthesis of Vitisins A and D Enabled by a Persistent Radical Equilibrium. J. Am. Chem. Soc. (2020). 142, 6499-6504, doi:10.1021/jacs.0c01714.
- [12] Romero, K. J. et al. Electrochemical Dimerization of Phenylpropenoids and the Surprising Antioxidant Activity of the Resultant Quinone Methide Dimers. Angew. Chem. Int. Ed. (2018). 57, 17125-17129, doi:10.1002/anie.201810870.
- [13] Keylor, M. H. et al. Synthesis of resveratrol tetramers via a stereoconvergent radical equilibrium. Science (2016). 354, 1260-1265, doi:10.1126/science.aaj1597.
- [14] Kärkäs, M. D., Porco, J. A. & Stephenson, C. R. J. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev. (2016). 116, 9683-9747, doi:10.1021/acs.chemrev.5b00760.
- [15] Douglas, J. J., Albright, H., Sevrin, M. J., Cole, K. P. & Stephenson, C. R. J. A Visible-Light-Mediated Radical Smiles Rearrangement and its Application to the Synthesis of a Difluoro-Substituted Spirocyclic ORL-1 Antagonist. Angew. Chem. Int. Ed. (2015). 54, 14898-14902, doi:10.1002/anie.201507369.
- [16] Matsuura, B. S. et al. A Scalable Biomimetic Synthesis of Resveratrol Dimers and Systematic Evaluation of their Antioxidant Activities. Angew. Chem. Int. Ed. (2015). 54, 3754-3757, doi:10.1002/anie.201409773.
- [17] Bos, P. H., Antalek, M. T., Porco, J. A. & Stephenson, C. R. J. Tandem Dienone Photorearrangement–Cycloaddition for the Rapid Generation of Molecular Complexity. J. Am. Chem. Soc. (2013). 135, 17978-17982, doi:10.1021/ja409992m.
- [18] Furst, L., Narayanam, J. M. R. & Stephenson, C. R. J. Total Synthesis of (+)-Gliocladin C Enabled by Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. (2011). 50, 9655-9659, doi:10.1002/anie.201103145.
- [19] Douglas, J. J., Sevrin, M. J. & Stephenson, C. R. J. Visible Light Photocatalysis: Applications and New Disconnections in the Synthesis of Pharmaceutical Agents. Org. Process Res. Dev. (2016). 20, 1134-1147, doi:10.1021/acs.oprd.6b00125.
- [20] Nguyen, J. D., Matsuura, B. S. & Stephenson, C. R. J. A Photochemical Strategy for Lignin Degradation at Room Temperature. J. Am. Chem. Soc. (2014). 136, 1218-1221, doi:10.1021/ja4113462.
- [21] Beatty, J. W. & Stephenson, C. R. J. Synthesis of (−)-Pseudotabersonine, (−)-Pseudovincadifformine, and (+)-Coronaridine Enabled by Photoredox Catalysis in Flow. J. Am. Chem. Soc. (2014). 136, 10270-10273, doi:10.1021/ja506170g.
- [22] Tucker, J. W., Zhang, Y., Jamison, T. F. & Stephenson, C. R. J. Visible-Light Photoredox Catalysis in Flow. Angew. Chem. Int. Ed. (2012). 51, 4144-4147, doi:10.1002/anie.201200961.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!
























No comments yet.