本文作者 alberto-caeiro
刘国生教授[1],博士毕业于上海有机化学研究所,师从陆熙炎院士,现为上海有机化学研究所研究员、教授。主要从事过渡金属催化的烯烃双官能团化反应和自由基反应,图片:实验室介绍。
个人经历
- 1991-1995,南京理工大学,学士;
- 1995-1999,大连化学物理研究所,硕士,指导教授:郑卓研究员;
- 1999-2002,上海有机化学研究所,博士,指导教授:陆熙炎院士;
- 2003-2005,Department of Chemistry, Lehigh University, Research Associate, With Prof. Li Jia;
- 2005–2007,Department of Chemistry, University of Wisconsin at Madison, Research Associate, With Prof. Shannon S Stahl;
- 2007-现在,上海有机化学研究所,教授
获奖经历
- 2011, The First Homogenous Catalysis Award;
- 2012, Thieme Chemistry Journal Award;
- 2012, National Funds for Distinguished Young Scientists;
- 2012, Asian Core Program Lectureship Award, Singapore;
- 2012, Asian Core Program Lectureship Award, Japan;
- 2012, 100 talents project, Chinese Academy of Sciences;
- 2014, Excellent Advisor for Ph. D Thesis, Chinese Academy of Sciences”
- 2016, Science and Technology Ministry of Youth Science and technology innovation leader;
- 2016, Zhu Liyuehua excellent teacher award, Chinese Academy of Science;
- 2016, National Youth Science and technology innovation leader, Ministry of Science and Technology of the People’s Republic of China;
- 2017, Shanghai Academic/Technology Research Leader, Science and Technology Commission of Shanghai Municipality;
- 2017, Shanghai Outstanding Leader, CPC Shanghai Municipal Commission;
- 2017, Excellent Advisor for Ph. D Thesis, Chinese Academy of Science;
- 2018, National ‘10000 talents projects’ science and technology innovation leader, Ministry of Science and Technology of the People’s Republic of China;
- 2018, Excellent teacher award, Chinese Academy of Science;
- 2018, Chinese Chemical Society-BASF youth Knowledge Innovation Award. Chinese Chemical Society of China.
工作介绍
1.过渡金属催化烯烃氧化双官能团化反应
金属Pd化学常见的催化循环都是钯(0)与钯(ll)或钯(ll)与钯(0)的循环,刘老师开发的烯烃氧化双官能团化反应利用高价钯利于还原消除的特性,实现了许多烯烃的双官能团化反应[2]。高价钯物种Pd(IV)的生成需要强的氧化剂,如PhI(OAc)2, Oxone, NXS和PhICl2等,这些强氧化剂在反应中会生成许多副产物,反应的原子经济性不高。过氧化氢是一类绿色的氧化剂,除了其经济易得外,其还原产物为水,于环境无害。刘老师利用过氧化氢作为氧化剂生成的高价钯物种,实现了烯烃的氧化胺化反应,如羟胺化[3a]、胺氯化[3b]、胺酰基化[3c,d]反应。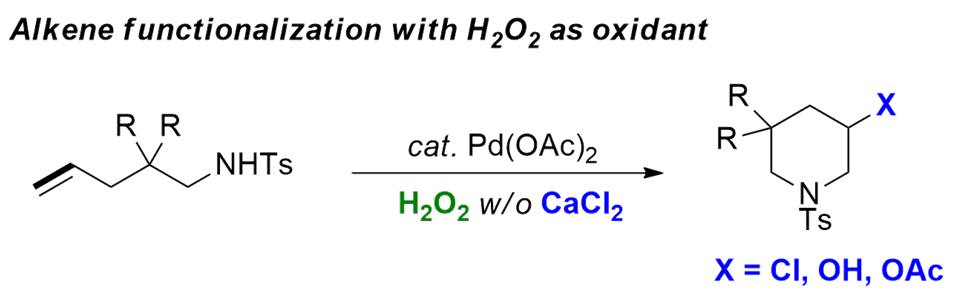
烯烃氧化双官能团化反应
- 过渡金属催化的氟化及相应转化
氟原子因其独特的电负性与原子大小,使含氟原子的分子在药物代谢中一般有良好的稳定性。将氟原子或含氟官能团引入分子中是具有挑战性且引人注目的一类反应。刘老师在氧化双官能团化反应的基础之上,将氧化剂和亲核氟源或含氟官能团引入氧化体系中,在温和条件下,实现了一系列氟源的引入,其中包括氟原子[4]、三氟甲基[5]、三氟甲氧基[6],三氟甲硫基[6]的引入。此外,含氟的芳杂环[7]也可得到。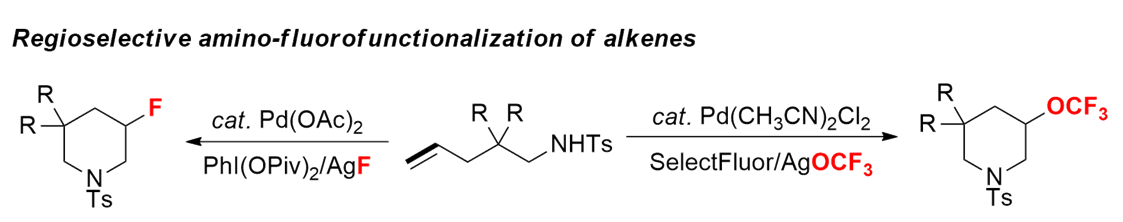
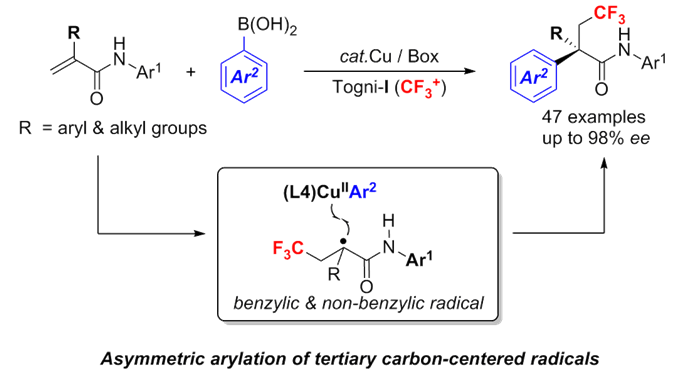
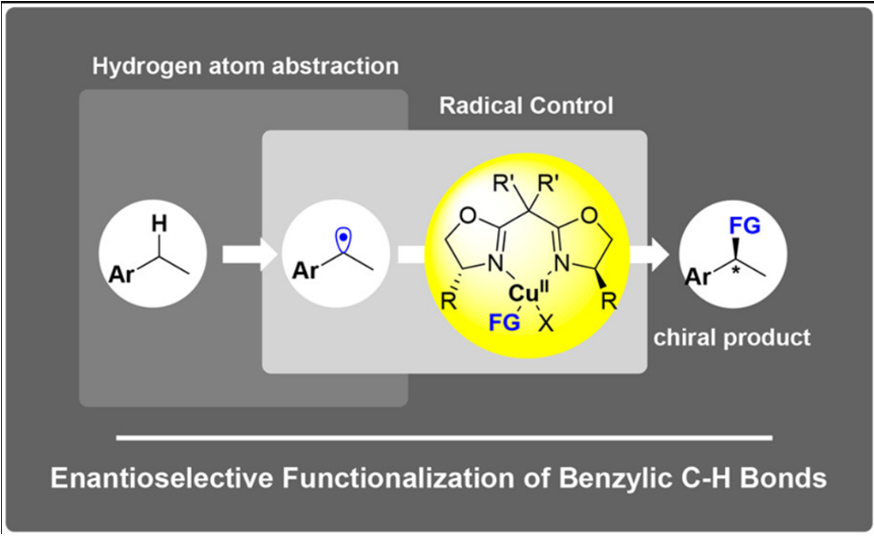
- 过渡金属催化的高选择性自由基反应
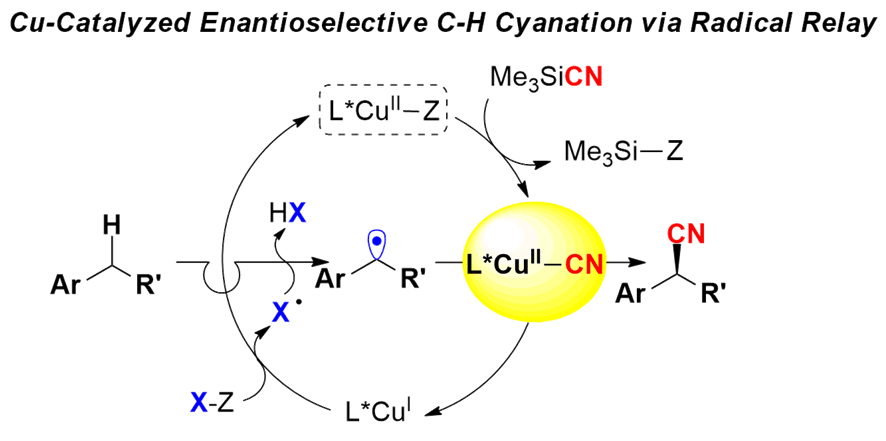
自由基化学在烯烃的双官能团化反应中是一种高效的方法,尤其是在分子内的反应中。而由于自由基的高反应活性,其选择性的控制则相较困难。刘老师则证实了通过可调节的过渡金属及配体复合物,可高效的控制自由基反应,从而实现了非对映选择性的氟化反应,对映选择性的氰化反应。此过程也可利用于C-H键的直接官能团化反应中[9a],刘老师在2016年的science上便报道了苄位的高对映选择性氰化反应[9b]。
Acc. Chem. Res. 2018, 51, 2036.
Science2016, 353, 1014
参考文献
- Liu G-S, Angew. Chem. Int. Ed., 2016, 55,15206. doi.org/10.1002/anie.201610019;
- Guoyin Yin, Xin Mu, Guosheng Liu,Acc. Chem. Res.,2016, 49, 2413.doi.org/10.1021/acs.accounts.6b00328;
- a. Zhu, Haitao; Chen, Pinhong; Liu, Guosheng, J. Am. Chem. Soc., 2014, 136, 1766.doi.org/10.1021/ja412023b; b. Yin, Guoyin; Wu, Tao; Liu, Guosheng, Chem. Eur. J., 2012, 18, 451. doi.org/10.1002/chem.201102776; c. Haitao Zhu, Pinhong Chen, Guosheng Liu, Org. Lett., 2015, 17, 1485. doi.org/10.1021/acs.orglett.5b00373; d. Xiaoxu Qi, Chaohuang Chen, Chuanqi Hou, Liang Fu, Pinhong Chen, Guosheng Liu, J. Am. Chem. Soc.,2018, 140, 7415. doi.org/10.1021/jacs.8b03767;
- a. Wu, Tao; Yin, Guoyin; Liu, Guosheng, J. Am. Chem. Soc., 2009, 131, 16354. doi.org/10.1021/ja9076588; b. Qiu, Shuifa; Xu, Tao; Zhou, Juan; Guo, Yinlong; Liu, Guosheng, J. Am. Chem. Soc., 2010, 132, 2856. doi.org/10.1021/ja909716k; c. Zhang, Zuxiao; Wang, Fei; Mu, Xin; Chen, Pinhong; Liu, Guosheng, Angew. Chem. Int. Ed., 2013, 52, 7549. doi.org/10.1002/anie.201301891; d. Zheliang Yuan, Hao-Yang Wang, Xin Mu, Pinhong Chen, Yin-Long Guo, Guosheng Liu, J. Am. Chem. Soc., 2015, 137, 2468. doi.org/10.1021/ja5131676; e. Xiaoxu Qi,Feng Yu, Pinhong Chen, Guosheng Liu, Angew. Chem. Int. Ed., 2017,56,12692. doi.org/10.1002/anie.201706401;
- a. Lianqian Wu, Fei Wang, Pinhong Chen, Guosheng Liu, J. Am. Chem. Soc.,2019, 141, 1887.doi.org/10.1021/jacs.8b13052; b. Liang Fu, Song Zhou, Xiaolong Wan, Pinhong Chen, Guosheng Liu, J. Am. Chem. Soc., 2018, 140, 10965. doi.org/10.1021/jacs.8b07436; c. Lianqian Wu, Fei Wang, Xiaolong Wan, Dinghai Wang, Pinhong Chen, Guosheng Liu, J. Am. Chem. Soc., 2017, 139, 2904.doi.org/10.1021/jacs.6b13299;
- a. Chaohuang Chen, Philipp Miro Pflüger, Pinhong Chen, Guosheng Liu, Angew. Chem. Int. Ed., 2019, 58, 2392. doi.org/10.1002/anie.201813591; b. Chaohuang Chen, Yixin Luo, Liang Fu, Pinhong Chen, Yu Lan,* Guosheng Liu, J. Am. Chem. Soc,, 2018,140,1207. doi.org/10.1021/jacs.7b11470; c. Chaohuang Chen, Pinhong Chen, Guosheng Liu, J. Am. Chem. Soc., 2015, 137, 15648. doi.org/10.1021/jacs.5b10971;
- a. Jiabin Xu, Pinhong Chen, Jingxing Ye, Guosheng Liu, Acta. Chim. Sinica., 2015, 73, 1294. DOI: 10.6023/A15030211; b. Jiabing Xu, Xin Mu, Pinhong Chen, Jinxing Ye, Guosheng Liu, Org. Lett., 2014, 16, 3942. doi.org/10.1021/ol501742a;
- a. Dinghai Wang, Zheliang Yuan, Qilun Liu, Pinhong Chen, Guosheng Liu, Chin. J. Chem. 2018, 36, 507. doi.org/10.1002/cjoc.201800016; b. Mu, Xin; Liu, Guosheng, Org. Chem. Front. 2014, 1, 430. DOI: 10.1039/C4QO00003J;
- a. Fei Wang, Pinhong Chen, Guosheng Liu,Acc. Chem. Res. 2018, 51, 2036. doi.org/10.1021/acs.accounts.8b00265; b. Wen Zhang,# Fei Wang,# Scott D. McCann, Dinghai Wang, Pinhong Chen, Shannon S. Stahl, Guosheng Liu,Science2016, 353, 1014. DOI: 10.1126/science.aaf7783.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!






















No comments yet.