本文投稿作者 alberto-caeiro
Ryan A. Shenvi,美国有机化学家,现就职于Scripps研究所。(图片:实验室主页)
经历
- 1999-2003 Pennsylvania State University B.S., (R.L. Funk),
- 2003-2008 The Scripps Research Institute Ph.D., (PhilS. Baran),
- 2008-2010 Harvard University NIH Postdoctoral Fellow, (E.J. Corey),
- 2010-2014 The Scripps Research Institute, Assistant Professor,
- 2014-present The Scripps Research Institute, Associate Professor.
获奖经历
- NDSEG Predoctoral Fellow, TSRI (2005-2008)
- Amgen Young Investigator Award (2013)
- Lesly Starr Shelton Award for Excellence in Graduate Studies (2007)
- Roche Excellence in Chemistry Award (2007)
- NIH Ruth L. Kirschstein Fellow, Harvard University (2008-2010)
- Eli Lilly New Faculty Award (2011)
- Boehringer Ingelheim New Investigator Award (2012)
- Baxter Foundation Young Investigator Award (2013)
- Alfred P. Sloan Research Fellow (2014)
- NSF Career Award (2014)
- BMS Unrestricted Grant in Synthetic Organic Chemistry Award (2014)
- Novartis Early Career Award (2014)
- Eli Lilly Grantee Award (2015)
- NPR Emerging Investigator (2016)
- Society of Synthetic Organic Chemistry (Japan) Lectureship Award (2017)
- Tetrahedron Young Investigator Award (2019)
研究概要
1.特殊药效团研究及天然产物合成
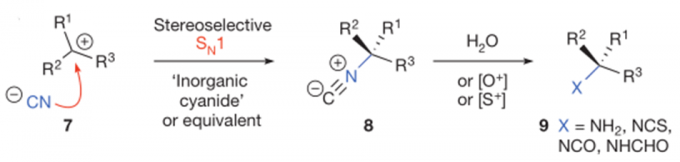
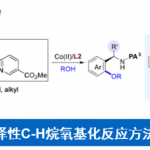
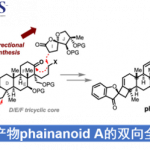
化学反应为理解小分子生物过程的机理提供了可行的方法,因此化学反应性的研究可为生物化学研究提供思路。Shenvi教授致力于含有特殊药效团的次级代谢产物的合成,这些天然产物在共价细胞活性(covalent cellular reactivity)上并没有被彻底解释清楚。天然产物包括以下几种:1)Nuphar二聚体【1】(Nuphar dimers),含有共价反应性的硫螺环药效团;2) asmarine生物碱【2】(asmarine alkaloids),其N-羟基二氮杂卓嘌呤可能结合金属;3)异氰萜类化合物【3,4】(isocyanoterpenes /ICTs),抗疟疾的海洋代谢产物,为研究此类产物,Shenvi教授开发了一种由四级醇经立体反转得到异氰的方法【4】。
- 氢转移的烯烃氢官能团化
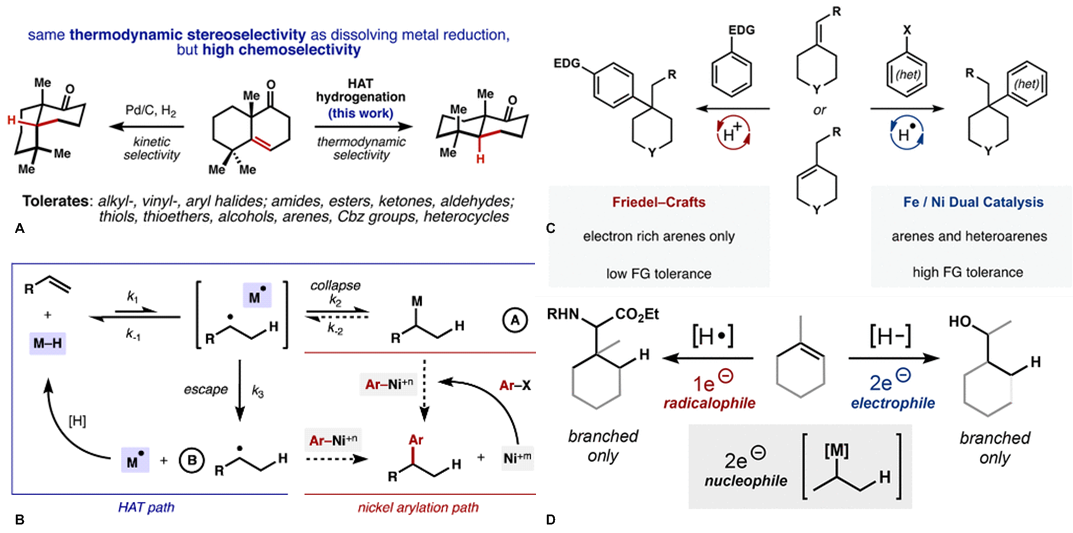
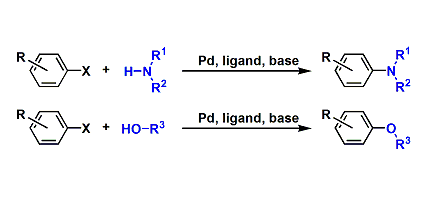
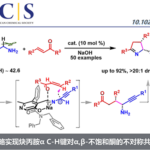
传统的烯烃氢官能团化需要强的Bronsted酸质子化形成瞬态高能量的碳正离子。于此相对应,由金属氢化物得到的烯烃氢官能团化反应是一类非传统的,经过低能量的碳自由基中间体的反应。Shenvi教授借鉴Halpern教授的氢转移反应,通过Co, Fe和Mn催化实现了烯烃的氢官能团化反应[5,6],并以此合成了许多难以合成的二级代谢产物(drimane, epoxyhumulene-II, α-funebrene, 7,20-diisocyanoadociane)。Shenvi教授还将金属氢化物催化的氢转移反应与镍催化的偶联反应结合起来,这种双金属催化的反应拓宽了底物的范围,预先官能团化的亲电试剂使反应有更好的应用前景,与其他金属联用的策略也使反应有更多的可能性。最近Shenvi教授已将双金属催化策略用于除镍外的金属,合成了支链选择的(branched-selective)烯烃氢官能团化产物。
A: 金属氢化物催化烯烃氢化反应;B, C: 双金属催化烯烃氢官能团化反应;D: 支链选择的烯烃与醛和亚胺反应
参考文献
- Jansen, D. J.; Shenvi, R. A. Synthesis of (–)-Neothiobinupharidine. J. Am. Chem. Soc.2013, 135, 1209.DOI: 10.1021/ja310778t;
- Wan, K. K.; Iwasaki, K.; Umotoy, J. C.; Wolan, D.; Shenvi, R. A. Nitrosopurines en route to Potent Asmarine Cytotoxins.Angew. Chem. Int. Ed.2015, 127, 2440.doi.org/10.1002/anie.201411493;
- a: Reiher, C. A.; Shenvi, R. A. Stereocontrolled Synthesis of Kalihinol C.J. Am. Chem. Soc.,2017, 139, 3647.DOI: 10.1021/jacs.7b01124; b: Pronin, S. V.; Shenvi, R. A. Synthesis of a Potent Antimalarial Amphilectene. J. Am. Chem. Soc.2012, 134, 19604.DOI: 10.1021/ja310129b; c: Pronin, S. V.; Shenvi, R. A. Synthesis of Highly Strained Terpenes by Nonstop Tail-to-Head Polycyclization. Nature Chem. 2012, 4, 915.doi.org/10.1038/nchem.1458;
- Pronin, S. V.; Reiher, C. A.; Shenvi, R. A. Stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles and amines. Nature. 2013, 501, 195.doi.org/10.1038/nature12472;
- a: Iwasaki, K.; Wan, K. K.; Oppedisano, A.; Crossley, S. W. M.; Shenvi R. A. J. Am. Chem. Soc.2014, 136, 1300. DOI: 10.1021/ja412342g; b: Green, S. A.; Matos, J. L. M.; Yagi, A.; Shenvi, R. A. J. Am. Chem. Soc. 2016, 138, 12779. DOI: 10.1021/jacs.6b08507; c: Green, S. A.; Vásquez-Céspedes, S.; Shenvi, R. A. J. Am. Chem. Soc. 2018, 140, 11317. DOI: 10.1021/jacs.8b05868; d:Matos, J.L.M.; Vásquez-Céspedes, S.; Gu, J.; Oguma, T.; Shenvi, R.A. J. Am. Chem. Soc. 2018, 140, 16976. DOI: 10.1021/jacs.8b11699;
- Crossley, S. W. M.; Martinez, R. M.; Obradors, C.; Shenvi, R. A. Chem. Rev. 2016, 116, 8912. DOI: 10.1021/acs.chemrev.6b00334.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!























No comments yet.