本文作者:alberto-caeiro
Timothy Jamison,美国有机合成化学家,现为美国麻省理工学院教授。图片:实验室介绍。
经历
- 1990 B.S. (Chemistry), University of California, Berkeley. (Prof. Henry Rapoport).
- 1991-1997 Ph.D. (Chemistry), Harvard University. (Prof. Stuart L. Schreiber).
- 1997-1999 Postdoctoral Fellow, Harvard University (Prof. Eric N. Jacobsen)
- 1999-2004 Assistant Professor, MIT, Department of Chemistry.
- 2002-2005 Paul M. Cook Career Development Chair.
- 2004-2006 Associate Professor (without tenure), MIT, Department of Chemistry.
- 2006-2009 Associate Professor, MIT, Department of Chemistry.
- 2009-present Professor, MIT, Department of Chemistry.
- 2015-present Robert R. Taylor Professor of Chemistry, MIT Department of Chemistry.
- 2015-2019 Department Head, MIT, Department of Chemistry.
- 2019-present Associate Provost, MIT.
获奖经历
- 2018 Change Maker Award, MIT Title IX.
- 2016 FP-Global Thinker of 2016.
- 2014 Council of Chemical Research Collaboration Award.
- 2013 Teaching Prize for Undergraduate Education, MIT School of Science.
- 2012 Fellow of the Royal Society of Chemistry.
- 2012 Royal Society of Chemistry Merck Award.
- 2011 Arthur C. Cope Scholar Award, American Chemical Society.
- 2006 JSPS Invitation Fellowship.
- 2004 Sloan Research Fellow.
- 2004 GlaxoSmithKline Scholar Award.
- 2003 Amgen Young Investigator Award.
- 2002 Boehringer Ingelheim New Investigator Award.
- 2001 National Science Foundation CAREER Award.
- 2000 3M Innovation Award.
- 1997-1999 Postdoctoral Fellow, Cancer Research Fund, Damon Runyon-Walter Winchell Foundation.
- 1991-1994 National Science Foundation Predoctoral Fellow.
- 1991-1993 Certificate of Distinction in Teaching, Harvard University (3 times).
- 1990-1991 Fulbright Fellow (Swiss Universities Grant).
- 1990 Graduated with High Honors (Chemistry), UC Berkeley.
- 1990 Saegebarth Prize (Undergraduate Research Excellence in Chemistry).
- 1990 Phi Beta Kappa.
- 1988-1989 President’s Undergraduate Fellow, UC Berkeley.
- 1985-1989 Chancellor’s Scholar, UC Berkeley.
- 1986-1989 Eastman Kodak Scholar.
工作介绍
1. Epoxide-opening cascades [1]
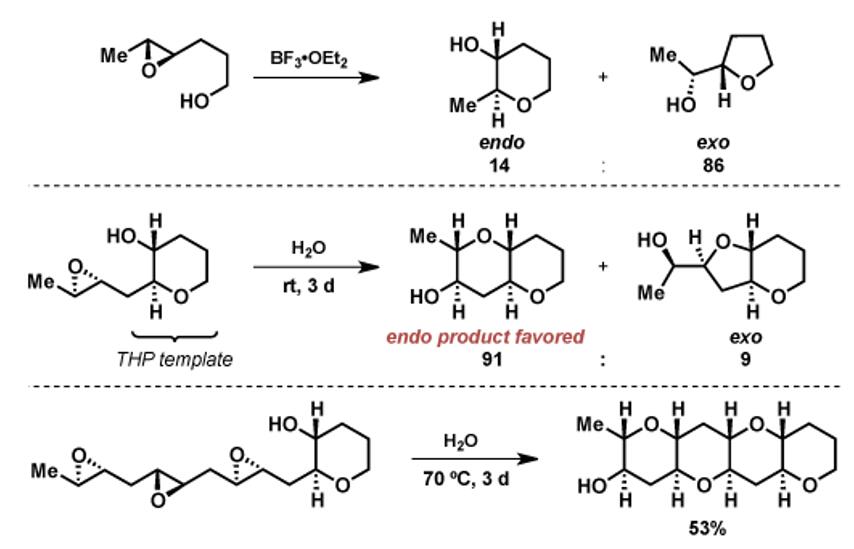
Jamison教授对环氧化物串联开环反应的发展与他们对天然产物梯形聚醚家族的兴趣相关。1984年,Nakanishi教授猜想此类天然产物是由环氧通过串联开环反应得到的,开环必须是以endo模式进行才能产生由连续C-C-O连接的trans-syn-trans环体系。但该猜想有一个缺点,即不论在酸性或碱性条件下,环氧开环反应的主要产物都是exo产物。
Jamison教授突破性的发现,当一个四氢吡喃环辅基(THP)在环氧开环反应前生成时,接下来的环氧开环反应在中性水溶液中加热即可发生,得到endo产物[2]。这是首次报道endo选择性的环氧串联开环反应,该方法可直接用于梯形聚醚天然产物家族的合成[d]。
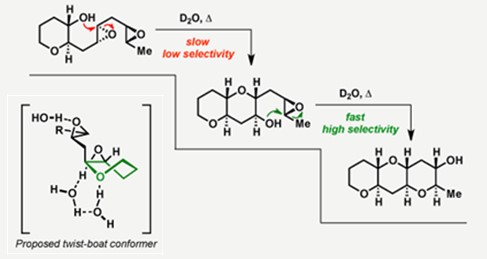
后续的机理研究表明,当使用其他模板(如环己烷,环氧己烷)代替THP时,反应还是得到exo选择性[3a]。同时,Jamison教授认为水在该反应中也有重要作用,猜测可能是在扭船式反应过渡态中起到稳定作用。动力学研究则表明,随着成环数目的增加,环氧开环反应的速率变得更快[3b]。
2. Ni-catalyzed C-C bonds formation reactions
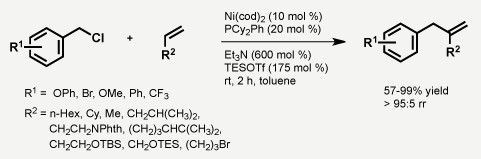
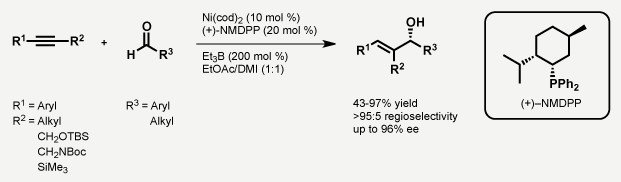
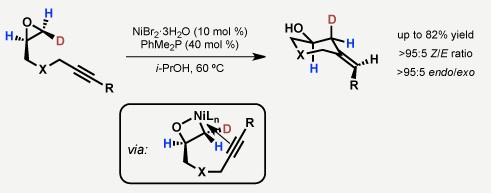
C-C成键反应是有机合成中最重要的反应。Jamison教授希望通过金属催化的C-C成键反应,将大宗原料如烯烃,环氧化物,醛等转化为更为复杂的结构。其主要工作包括如下两个方面:1. α-烯烃的(杂)苄基化,(杂)芳基化反应合成1,1-二取代端烯[4];2. 醛[5],环氧化物[6]和炔烃,烯烃的还原偶联反应。
Nickel-catalyzed intermolecular benzylation of α-olefin
Reductive coupling reactions between alkynes and aldehydes
Reductive coupling reactions between alkynes and epoxides
3. Continuous-flow chemistry
近年来,Jamison教授主要研究方向是流动化学,实现了许多流动化学反应。他们感兴趣的反应有以下特征:(1) fast and/or exothermic; (2) mixing- or transport-dependent (e.g. gas-liquid); (3) pathlength dependent (e.g. photochemical, electrochemical); (4) involve unstable or hazardous intermediates; and (5) are “slow” under typical conditions. 以下为部分实例。
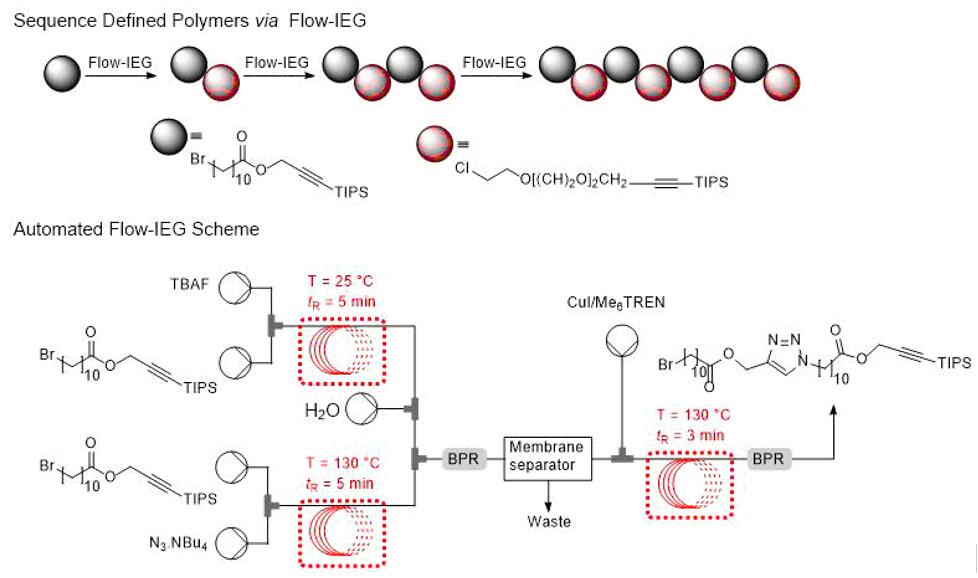
Synthesis of sequence-defined polymers via Flow-IEG (iterative exponential growth) [7]
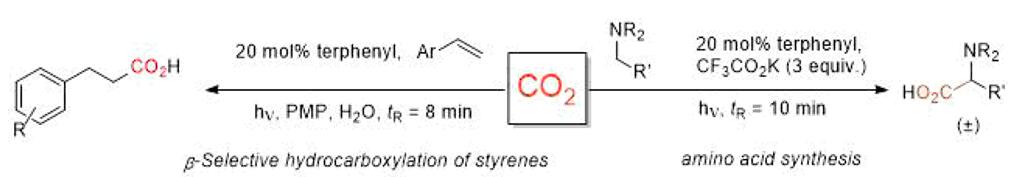
Synthesis of amino acids and β-hydrocarboxylated styrenes via photoredox activation of CO2 [8]
通过使用流动化学的方法,他们还和成了一系列的分子。如Linezolid[9a]和Dolutegravir[9b],以及下图中所示的分子。
Targeted synthesis in flow chemistry
参考文献
- [1] For a review, see: Jamison, T. F. Chem. Soc. Rev. 2009, 38, 3175. DOI: 10.1039/B816697H.
- [2] a. Jamison, T. F. J. Am. Chem. Soc. 2006, 128, 1056. DOI: 10.1021/ja057973p; b. Jamison, T. F. Science 2007, 317,1189. DOI: 10.1126/science.1146421; c. Jamison, T. F. Chem. Eur. J. 2013, 19, 10004. DOI: 10.1002/chem.201300845; d. Jamison, T. F. J. Am. Chem. Soc. 2019, 141, 11239. DOI: 10.1021/jacs.9b04696.
- [3] a. Jamison, T. F. J. Am. Chem. Soc. 2009, 131, 6383. DOI: 10.1021/ja9004909; b. Jamison, T. F. J. Am. Chem. Soc. 2011, 133, 1902. DOI: 10.1021/ja1088748.
- [4] a. Jamison, T. F. J. Am. Chem. Soc. 2011, 133, 19020. DOI: 10.1021/ja209235d; b. Jamison, T. F. J. Am. Chem. Soc. 2013, 135, 1585. DOI: 10.1021/ja3116718; c. Jamison, T. F. Angew. Chem. Int. Ed. 2014, 53, 1858. DOI: 10.1002/anie.201308391; d. Jamison, T. F. J. Am. Chem. Soc. 2015, 137, 9531. DOI: 10.1021/jacs.5b05597.
- [5] a. Jamison, T. F. J. Am. Chem. Soc. 2003, 125, 3442. DOI: 10.1021/ja034366y; b. Jamison, T. F. J. Am. Chem. Soc. 2009, 131, 665. DOI: 10.1021/ja900701g.
- [6] a. Jamison, T. F. J. Am. Chem. Soc. 2003 125, 8076. DOI: 10.1021/ja0361401; b. Jamison, T. F. Org. Lett. 2011, 13, 4140. DOI: 10.1021/ol201702a.
- [7] a. Jamison, T. F. Nat. Chem. 2015, 7, 810. DOI: 10.1038/nchem.2346; b. Jamison, T. F. Proc. Natl. Acad. Sci. USA, 2015, 112, 10617. DOI: 10.1073/pnas.1508599112.
- [8] a. Jamison, T. F. J. Am. Chem. Soc. 2017, 139, 13969. DOI: 10.1021/jacs.7b05942; b. Jamison, T. F. Nat. Chem. 2017, 9, 453. DOI: 10.1038/nchem.2690.
- [9] a. Jamison, T. F. Angew. Chem Int. Ed. 2019, 58, 7678. DOI: 10.1002/anie.201901814; b. Jamison, T. F. Angew. Chem. Int. Ed. 2018, 57, 7181. DOI: 10.1002/anie.201802256.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!























No comments yet.