本文作者:杉杉
导读
近日,西班牙加泰罗尼亚化学研究所Ruben Martin教授课题组在J. Am. Chem. Soc.上发表论文,报道了一种在CO2(1 bar)氛围中,通过Ni催化实现N-取代氮丙啶(aziridines)的还原羧化反应,从而获得一系列β-氨基酸衍生物。同时,该反应具有温和的反应条件、出色的化学和区域选择性等特点。
Ni-Catalyzed Carboxylation of Aziridinesen Route to β‑Amino Acids
Jacob Davies, Daniel Janssen-Muller, Dmitry P. Zimin, Craig S. Day, Tomoyuki Yanagi, Jonas Elfert, and Ruben Martin*
J.Am. Chem. Soc.ASAP DOI:10.1021/jacs.1c01916
正文
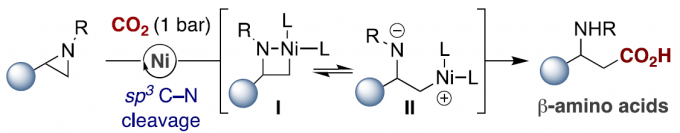
有机卤化物与二氧化碳的催化还原羧化反应,是合成羧酸化合物的有效策略。尽管已经取得一定的进展,但寻找一种更为高效且操作简单的策略,则面临众多挑战。受Hillhouse和Wolfe等[1]开创性研究的启发,其他研究者也实现了氮丙啶与有机金属试剂的催化芳基化/烷基化反应[2],邻位导向C-H官能化[3]或通过sp3 C-N裂解的还原途径[4](Scheme 1, top)。然而,在还原偶联反应中通过芳基以外的底物,合成具有价值的β-官能化胺化合物仍具有难度。在此,作者设想,是否可设计一种新型催化策略,可通过I/II以位点选择的方式将CO2引入到氮丙啶中,从而直接合成具有价值的β-氨基酸分子,可避免了有害物质的使用,如重氮化合物、氰化物、一氧化碳等(Scheme 1, bottom)。
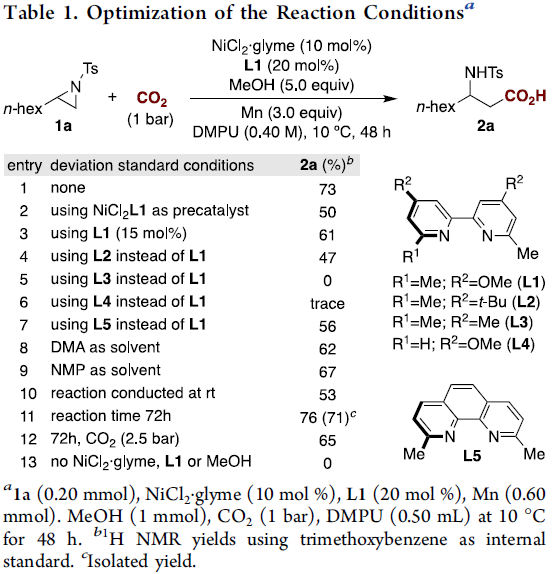
首先,作者以1a作为模型底物,进行了相关还原反应条件的筛选(Table 1)。反应的最佳条件为:在1 bar的CO2下,以10 mol%的NiCl2·glyme为催化剂,20 mol%的L1为配体,3当量Mn粉为还原剂,同时加入5当量的甲醇,可在DMPU溶剂中反应,获得73%收率的产物2a。
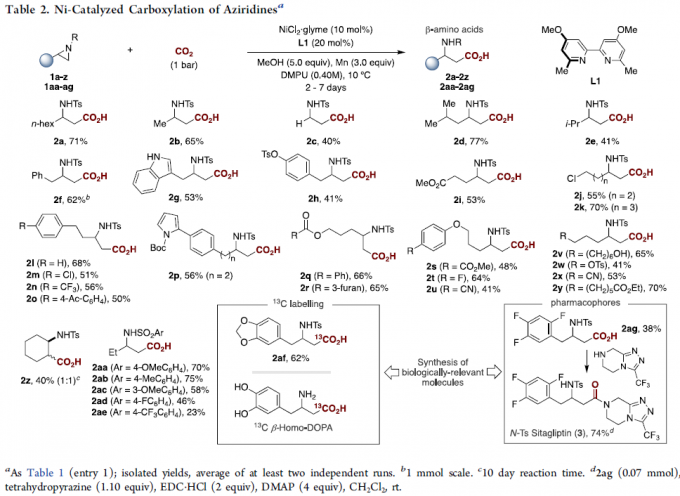
在获得上述最佳反应条件后,作者开始对底物1进行了扩展(Table 2)。首先,具有不同β位取代的底物,均可顺利反应,获得相应的氨基酸产物2a–2i。值得注意的是,在β位具有空间位阻大的底物(1e)或无取代的底物(1c),收率偏低。其次,一系列活性的官能团均可耐受,如酯(2i,2q,2r,2s,2y),酮(2o)和腈(2u,2x)。值得注意的是,含氮或氧杂环的底物,不会干扰氮丙啶骨架(2g,2p,2r)的有效羧化。同时,具有甲苯磺酸芳基化物(2h),氯化物(2m)和烷基卤化物(2j,2k)或磺酸盐(2w)取代的底物,也可有效的进行羧化反应,为进一步偶联反应提供了多种可能。2,3-二取代的氮丙啶类化合物也可获得较低收率和非对映选择性的产物2z。此外, Ts的苯环上具有不同取代基时,均取得良好的结果,获得产物2aa–2ae。然而,2-芳基氮丙啶则与体系不相容。值得注意的是,该策略成功应用于生物活性分子的合成,如2af和2ag。
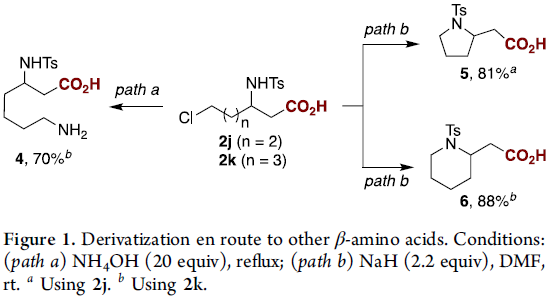
紧接着,作者对反应的实用性进行了研究(Figure 1)。在path a-b中,以2j和2k为底物,通过一步操作即可合成β-高赖氨酸(homolysine,4),β-高脯氨酸(homoproline,5)和哌啶-2-基-乙酸(6)。
随后,作者对反应机理进行了研究(Scheme 2)。首先,当以Ni(L5)2为催化剂,与不同当量的MeOH和Mn反应时,只有在MeOH和Mn均存在的条件下,Ni(L5)2才具有催化作用,从而表明Ni(I)配合物参与催化循环的过程(Scheme 2, top)。虽然未能阐明MeOH的作用,但它可能会促进和/或稳定II型中间体。同时,对于1l的反应监控发现,反应过程中形成了2l、TsNH2、4-苯基丁烷-2-酮和4-苯基-1-丁烯的混合物,从而表明反应涉及烷基镍配合物的β-氢化或脱氨基途径。同时,通过1ab与Ni(L5)2反应中获得二氮杂镍环戊烯(Ni-1)中间体,进一步证明了上述的观点。此外,也有可能通过原位生成的金属烯胺III亲核加到CO2上,但通过将对映体(S)-1f转化为7且手性保持完整,从而反对了该过程。最后,作者将注意力转向研究反应的立体化学过程。反式1a–d1经羧化反应、还原反应和环化反应,获得顺式/反式比例4:1的环化产物9。虽然主要产物可能是通过将Ni(0)经SN2型插入氮丙啶骨架来解释,但反式异构体的存在以及2z中非对映体的缺乏,表明其他可能的途径可能会起作用。这些结果是否表明单电子转移过程的参与或经由自由基中间体的重组,则需进一步的研究。

总结
西班牙加泰罗尼亚化学研究所Ruben Martin教授课题组报道了一种温和的选择性催化策略,可从易得的氮丙啶直接合成具有价值的β-氨基酸衍生物。同时,该反应具有反应条件温和、底物范围广泛、化学和区域选择性出色等特点。值得注意的是,MeOH作为添加剂、Mn作为还原剂以及配体的使用,对于反应至关重要。
参考文献
[1] (a) Lin, B. L.; Clough, C. R.; Hillhouse, G. L. Interactions of Aziridines with Nickel Complexes: Oxidative-Addition and Reductive-Elimination Reactions that Break and Make C−N Bonds.
J. Am. Chem. Soc. 2002,
124, 2890. (b) Ney, J. E.; Wolfe, J. P. Synthesis and Reactivity of Azapalladacyclobutanes.
J. Am. Chem. Soc. 2006,
128, 15415.
[2] For metal-catalyzed cross-coupling reactions of aziridines and organozinc reagents, see: (a) Huang, C.-Y. D.; Doyle, A. G. Nickel-Catalyzed NegishiAlkylations of Styrenyl Aziridine.
J. Am. Chem. Soc. 2012,
134, 9541. (b) Nielsen, D. K.; Huang, C.-Y.; Doyle, A. G. Directed Nickel-Catalyzed Negishi Cross Coupling of Alkyl Aziridines.
J. Am. Chem. Soc. 2013,
135, 13605. (c) Jensen, K. L.; Standley, E. A.; Jamison, T. F. Highly Regioselective Nickel-Catalyzed Cross-Coupling of N-Tosylaziridines and Alkylzinc Reagents.
J. Am. Chem. Soc. 2014,
136, 11145. For the utilization of organoboron reagents, see: (d) Duda, M. L.; Michael, F. E. Palladium-Catalyzed Cross-Coupling of N-Sulfonylaziridines with Boronic Acids.
J. Am. Chem. Soc. 2013,
135, 18347. (e) Takeda, Y.; Ikeda, Y.; Kuroda, A.; Tanaka, S.; Minakata, S. Pd/NHC-Catalyzed Enantiospecific and Regioselective Suzuki−MiyauraArylation of 2-Arylaziridines: Synthesis of Enantioenriched 2-Arylphenethylamine Derivatives.
J. Am. Chem. Soc. 2014,
136, 8544. (f) Takeda, Y.; Kuroda, A.; Sameera, W. M. C.; Morokuma, K.; Minakata, S. Palladium-catalyzed regioselective and stereo-invertive ring-opening borylation of 2-arylaziridines with bis(pinacolato)diboron: experimental and computational studies.
Chem. Sci. 2016,
7, 6141. (g) Takeda, Y.; Matsuno, T.; Sharma, A. K.; Sameera, W. M. C.; Minakata, S. Asymmetric Synthesis of β2-Aryl Amino Acids through Pd-Catalyzed Enantiospecific and Regioselective Ring-Opening Suzuki−MiyauraArylation of Aziridine-2-carboxylates.
Chem. – Eur. J. 2019,
25, 10226. (h) Yu, X.-Y.; Zhou, Q.-Q.; Wang, P.-Z.; Liao, C.-M.; Chen, J.-R.; Xiao, W.-J. Dual Photoredox/Nickel-Catalyzed Regioselective Cross-Coupling of 2-Arylaziridines and Potassium Benzyltrifluoroborates: Synthesis of β-Substitued Amines.
Org. Lett. 2018,
20, 421.
[3] For selected references: (a) Li, X. W.; Yu, S. J.; Wang, F.; Wan, B. S.; Yu, X. Z. Rhodium(III)-Catalyzed C−C Coupling between Arenes and Aziridines by C−H Activation.
Angew .Chem. Int. Ed. 2013,
52, 2577. (b) Gao, K.; Paira, R.; Yoshikai, N. Cobalt-Catalyzed ortho-C−H Alkylation of 2-Arylpyridines via Ring-Opening of Aziridines.
Adv. Synth. Catal. 2014,
356, 1486. (c) Zhou, K.; Zhu, Y.; Fan, W.; Chen, Y.; Xu, X.; Zhang, J.; Zhao, Y. Late-Stage Functionalization of Aromatic Acids with Aliphatic Aziridines: Direct Approach to Form β-Branched Arylethylamine Backbones.
ACS Catal. 2019,
9, 6738.
[4] (a) Woods, B. P.; Orlandi, M.; Huang, C.-Y.; Sigman, M. S.; Doyle, A. G. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling of StyrenylAziridines.
J. Am. Chem. Soc. 2017,
139, 5688. (b) Steiman, T. J.; Liu, J.; Mengiste, A.; Doyle, A. G. Synthesis of β-Phenethylamines via Ni/Photoredox Cross-Electrophile Coupling of Aliphatic Aziridines and Aryl Iodides.
J. Am. Chem. Soc. 2020, 142, 7598.
























No comments yet.