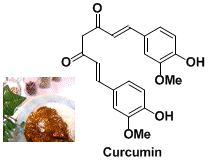
姜黄素(curcumin)是一种从姜黄(Curcuma longa)根茎中提取得到的黄色色素,从结构来看是一种多酚类化合物。
- 历史・用途
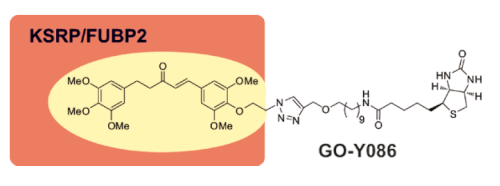
有数据表明,印度人由于常年吃咖喱,患癌几率比不吃咖喱的要低,而咖喱的黄色就是由来于姜黄素。姜黄素具有抗氧化、抗癌作用。早在1988年开始,美国国立癌症研究所就开始了对姜黄素的抗癌研究。近年来,随着荧光标记技术的发展,通过合成姜黄素的分子探针(GO-Y086)发现了姜黄素的抗癌受体蛋白FUBP,为其抗癌机制提供了依据(1)
同时,不仅仅是抗癌活性,最近又发现该物质显示出抗阿尔茨海默的效果。
由于姜黄素含有多种有益活性,因此含有姜黄素的多种健康食品如雨后春笋。比如说姜黄素的解酒饮料,这是因为姜黄素可以活化胆汁的分泌,维持肝脏整体的良好的功能。
- 关联文献
(1) “KSRP/FUBP2 Is a Binding Protein of GO-Y086, a Cytotoxic Curcumin Analogue”
Yamakoshi, H.; Kanoh, N.; Kudo, C.; Sato, A.; Ueda, K.; Muroi, M.; Kon, S.; Satake, M.; Ohori, H.; Ishioka, C. ACS Med. Chem. Lett. 2010, 1, 273-276. DOI:?10.1021/ml1000454
Bis(arylmethylidene)acetone derivatives are an important class of curcumin analogues that exhibit various biological and pharmacological activities. We herein report that GO-Y086, a biotinylated bis(arylmethylidene)acetone, inhibits cancer cell growth. We also show that GO-Y086 specifically interacts with the nuclear protein KSRP/FUBP2 by covalent modification. GO-Y086 markedly suppresses the expression of the c-Myc protein, which plays an important role in cellular proliferation and whose expression is regulated by KSRP/FUBP2.
(2) “Structure–activity relationship of C5-curcuminoids and synthesis of their molecular probes thereof”
Yamakoshi, H.; Ohori, H.; Kudo, C.; Sato, A.; Kanoh, N.; Ishioka, C.; Shibata, H.; Iwabuchi, Y. Bioorg. Med. Chem. 2010, 18, 1083-1092. DOI:10.1016/j.bmc.2009.12.045
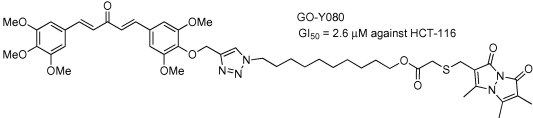
A series of novel analogues of 1,5-bis(4-hydroxy-3-methoxyphenyl)-penta-(1E,4E)-1,4-dien-3-one (C5-curcumin), which is a natural analogue of curcumin isolated from the rhizomes of Curcuma domestica Val. (Zingiberacea), were synthesized and evaluated for their cytotoxicities against human colon cancer cell line HCT-116 to conclude the SAR of C5-curcuminoids for further development of their use in cancer chemotherapy: (1) Bis(arylmethylidene)acetone serves as a promising skeleton for eliciting cytotoxicity. (2) The 3-oxo-1,4-pentadiene structure is essential for eliciting cytotoxicity. (3) As for the extent of the aromatic substituents, hexasubstituted compounds exhibit strong activities, in which 3,4,5-hexasubstitution results in the highest potency. (5) The symmetry between two aryl rings is not an essential requirement for bis(arylmethylidene)acetones to elicit cytotoxicity. (6) para-Positions allows the installation of additional functional groups for use as molecular probes. By taking advantage of the SAR diagram, we have elaborated several advanced derivatives having GI50 of single-digit micromolar potencies that will function as molecular probes to target and/or report key biomolecules interacting with curcumin and C5-curcumin.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!













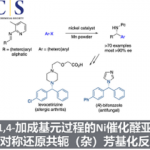
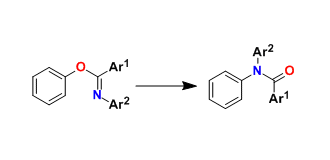

No comments yet.