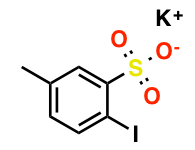
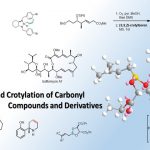
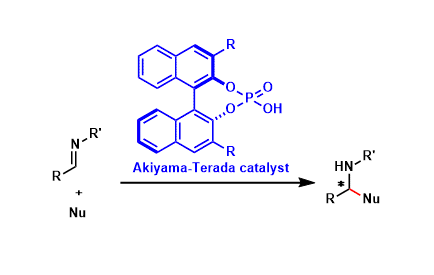
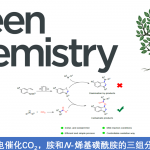
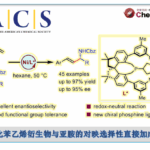
pre-MIBSK是名古屋大学的石原教授课题组开发的含有高价碘的醇氧化催化剂。与Dess-Martin试剂相比,只需要催化量就可以在体系中产生活性种,因此在安全性与成本上更佳。该催化剂对仲醇的氧化也很有效,并且对于伯醇的氧化来说,通过调节协同催化剂的Oxone®的量,可以选择性的得到醛或者羧酸。
〈参考文献〉
Uyanik, M., Akakura, M., Ishihara, K. : J. Am. Chem. Soc., 2009, 131, 251. DOI: 10.1021/ja807110n
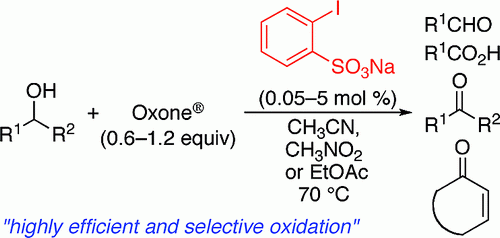
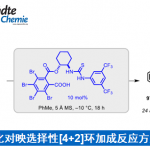
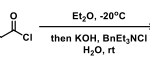
[2] “2-Iodoxy-5-Methylbenzenesulfonic Acid-Catalyzed Selective Oxidation of 4-Bromobenzyl Alcohol to 4-Bromobenzaldehyde or 4-Bromobenzoic Acid with Oxone”Electron-donating group-substituted 2-iodoxybenzoic acids (IBXs) such as 5-Me-IBX (1g), 5-MeO-IBX (1h), and 4,5-Me2-IBX (1i) were superior to IBX 1a as catalysts for the oxidation of alcohols with Oxone (a trademark of DuPont) under nonaqueous conditions, although Oxone was almost insoluble in most organic solvents. The catalytic oxidation proceeded more rapidly and cleanly in nitromethane. Furthermore, 2-iodoxybenzenesulfonic acid (IBS, 6a) was much more active than modified IBXs. Thus, we established a highly efficient and selective method for the oxidation of primary and secondary alcohols to carbonyl compounds such as aldehydes, carboxylic acids, and ketones with Oxone in nonaqueous nitromethane, acetonitrile, or ethyl acetate in the presence of 0.05−5 mol % of 6a, which was generated in situ from 2-iodobenzenesulfonic acid (7a) or its sodium salt. Cycloalkanones could be further oxidized to α,β-cycloalkenones or lactones by controlling the amounts of Oxone under the same conditions as above. When Oxone was used under nonaqueous conditions, Oxone wastes could be removed by simple filtration. Based on theoretical calculations, we considered that the relatively ionic character of the intramolecular hypervalent iodine−OSO2 bond of IBS might lower the twisting barrier of the alkoxyperiodinane intermediate 16.
Uyanik, M., Ishihara, K. Org. Synth. 2012, 89, 105. DOI:10.15227/orgsyn.089.0105
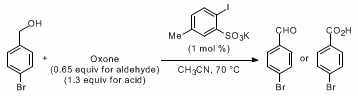
催化循环
参考文献
- M. Uyanik, M. Akakura, K. Ishihara: J. Am. Chem. Soc., 131, 251 (2009).
- M. Uyanik, K. Ishihara : Org. Synth., 89, 105 (2012).
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!














No comments yet.