Erick M. Carreira教授1963年生于哈瓦那、古巴(Havana Cuba)。现为苏黎世联邦理工学院有机化学教授,ACS旗下杂志Organic Letter主编, (图片来自:Org Lett), 自2021年起担任J. Am. Chem. Soc.主编。
经历
- 1984年 University of Illinois at Urbana-Champaign, B.S. Scott E. Denmark;
- 1990年 Harvard University, Ph.D. David A. Evans;
- 1992年 California Institute of Technology, Postdoctoral,Peter Dervan;
- 1996年California Institute of Technology,助理教授
- 1997年California Institute of Technology, 教授
- 1998年-现在Swiss Federal Institute of Technology Zurich, 教授
获奖经历
- American Chemical Society Award in Pure Chemistry;
- Nobel Laureate Signature Award;
- Fresenius Award;
- David and Lucile Packard Foundation Fellowship in Science;
- Alfred P. Sloan Fellowship;
- Camille and Henry Dreyfus Teacher Scholar Award;
- Merck Young Investigator Award;
- Eli Lilly Young Investigator Award;
- Pfizer Research Award, National Science Foundation CAREER Award;
- Arnold and Mabel Beckman Young Investigator Award;
- Camille and Henry Dreyfus New Faculty Award;
- the Associated Students of the California Institute of Technology Annual Award in Teaching;
- Richard M. Badger Award in Teaching;
研究概览
1.生物活性分子的全合成
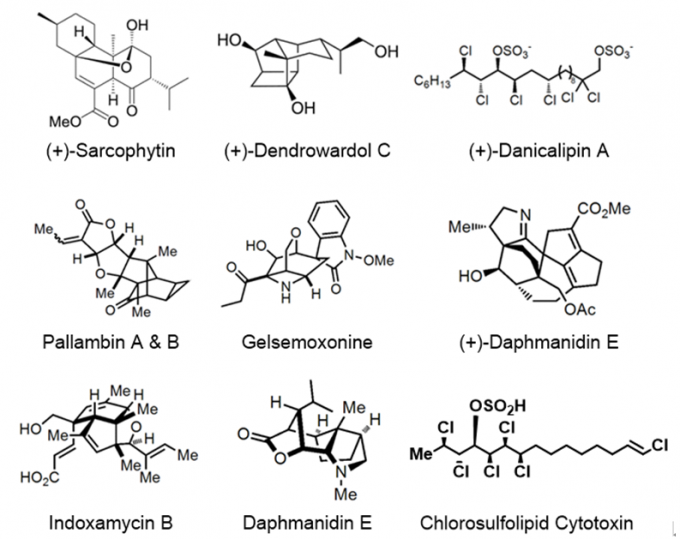
Erick M. Carreira教授一直致力于有生物活性和结构复杂的天然产物分子高效全合成,课题组至今已合成许多天然产物,图一为代表性分子的合成。其中,Chlorosulpholipid Cytotoxin的合成工作发表于顶级期刊Nature【1】。
图1:Representative Synthesis
2.合成策略的开发
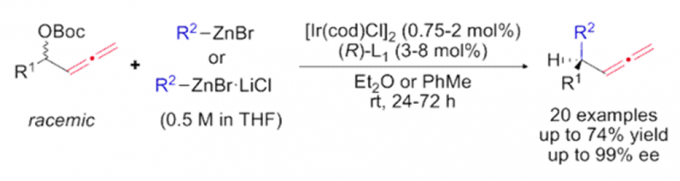
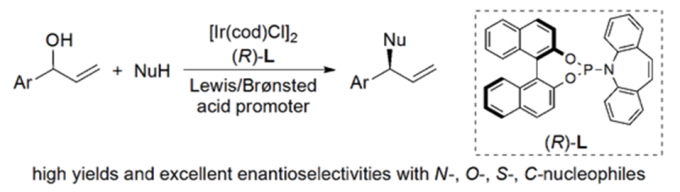
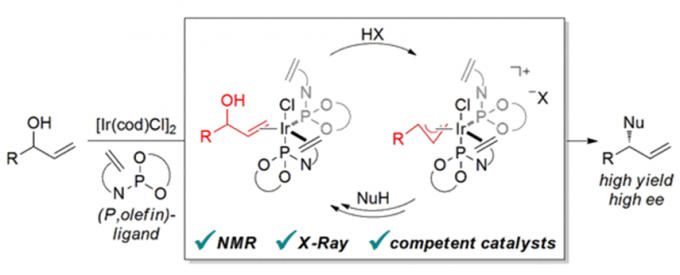
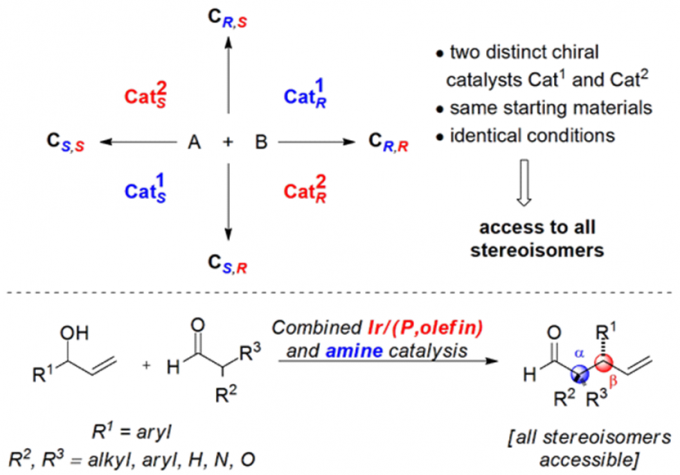
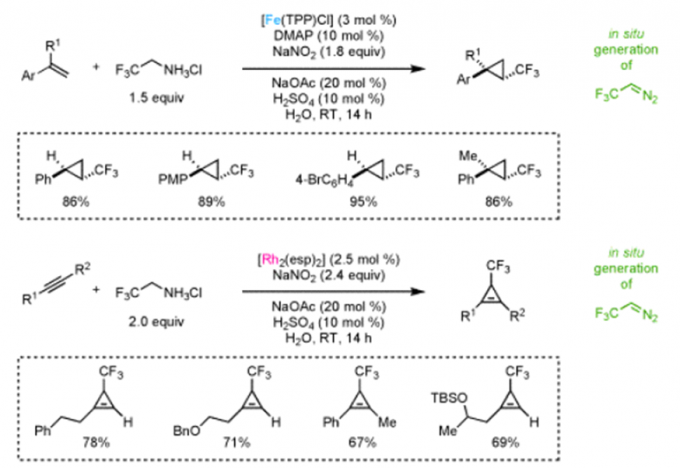
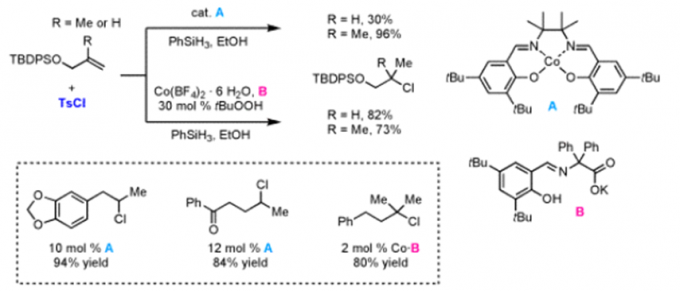
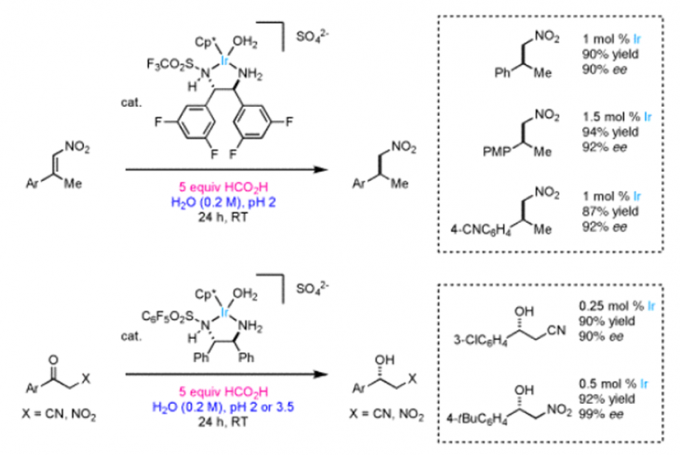
Erick M. Carreira教授的合成方法学主要包括一下方面:联烯不对称烷基化反应{2}(图2a),不对称的烯丙基取代反应及其机理研究[3,4](图2b, c),对映选择性和非对映选择性的双催化剂在手性合成中的应用[5](图2d),非活化双键的氢氯化反应[6](图2e),环丙烷和环丙烯的不对称三氟甲基化反应[7](图2f),水相中的不对称转移氢化反应[8](图2g)。
a: Iridium-Catalyzed Enantioconvergent Allenylic Alkylation
b: Iridium-Catalyzed Enantioconvergent Allylic Substitution
c: Mechanistic Studies Regarding the Iridium Catalyzed Enantioselective Allylic Substitution
d: Enantio- and Diastereodivergent Dual Catalysis and Its Application
e: Trifluoromethylation of Cyclopropanes & Cyclopropenes
f: Hydrochlorination of Unactivated Olefins
g: Asymmetric Transfer Hydrogenations in Water
图2:Representative Catalytic Methods
- 药物化学
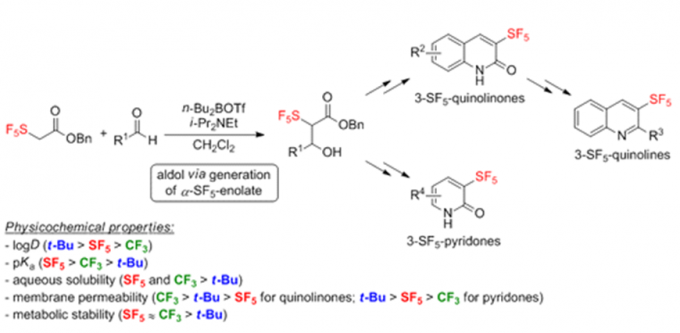
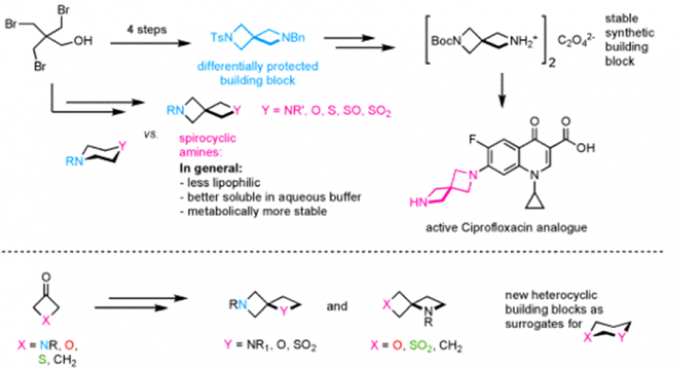
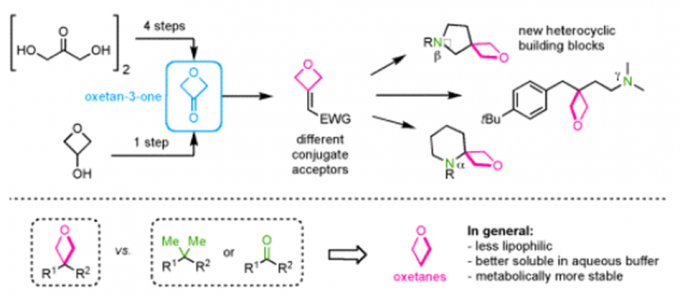
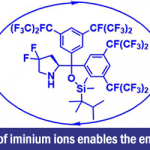
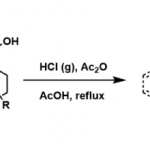
Erick. M. Carreira教授在药物活性片段及活性方面也有许多研究,活性分子的氟化,杂环SF5化反应(图3a),螺环胺(图3b),氧杂环丁烷(图3c)。
a: 杂环SF5化反应
b:螺环胺合成
c:氧杂环丁烷合成
图3:Representative Workin Medicinal Chemistry
参考文献
1.C. Nilewski, R.W. Geisser, E. M. Carreira, Nature 2009, 547, 573.doi.org/10.1038/nature07734.
- D.A. Petrone, M. Isomura, I. Franzoni, S.L. Rössler, E.M. Carreira, J. Am. Chem. Soc. 2018, 140,4697. DOI: 10.1021/jacs.8b01416.
- J. Y. Hamilton, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2014, 136, 3006. DOI: 10.1021/ja412962w.
- S.L. Rössler, S.Krautwald, E.M. Carreira, J. Am. Chem. Soc. 2017, 139, 3603-3606.DOI: 10.1021/jacs.6b12421.
- a: S. Krautwald, D. Sarlah, M. A. Schafroth, E. M. Carreira, Science, 2013, 340, 1065.DOI: 10.1126/science.1237068; b: (Perspective) S. Krautwald, E.M. Carreira, J. Am. Chem. Soc. 2017, 139, 5627-5639. DOI: 10.1021/jacs.6b13340.
- a: B. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 938. doi.org/10.1002/anie.200905573;b: B. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 4294.doi.org/10.1002/anie.201000787;
- B. Gaspar, E. M. Carreira, Angew. Chem. Int. Ed. 2008, 47, 5758. doi.org/10.1002/anie.200801760.
- O. Soltani, M. A. Ariger, H. Vázquez-Villa, E. M. Carreira, Org. Lett. 2010, 12, 2893. DOI: 10.1021/ol1008894.
链接
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!
























No comments yet.