Varinder Kumar Aggarwal(1961年1月1日——),现为英国布里斯托大学(University of Bristol)教授。图片:实验室主页。
经历
- 1980–1983, University of Cambridge, B. A.;
- 1983–1986, University of Cambridge, Ph. D., Advisor: Prof. Stuart Warren;
- 1986–1988, Columbia University, Postdoctoral position, Advisor: Prof. Gilbert Stork;
- 1988–1991, University of Bath, Lecturer(讲师) in Chemistry;
- 1991–1995, University of Sheffield, Lecturer in Chemistry;
- 1995–1997, University of Sheffield, Reader(副教授) in Chemistry;
- 1997–2000, University of Sheffield, Professor in Chemistry;
- 2000 – present, University of Bristol, Professor in Synthetic Chemistry;
- 2019 – present, University of Bristol, Alfred Capper Pass Professor of Chemistry.
获奖经历
- 1986–1988, Harkness Fellowship;
- 1996, Pfizer Academic Award;
- 1996, Glaxo Wellcome Academic Award;
- 1996, Zeneca Academic Award;
- 1997, RSC Hickinbottom Fellowship;
- 1997–1998, Nuffield Foundation Fellow;
- 1998, Pfizer Academic Award;
- 1999, RSC Corday Morgan Prize and Medal;
- 1999–2000, Novartis Lecturship;
- 1999–2000, Liebigs Lecturship;
- 2003, RSC Green Chemistry Award;
- 2004, Zeneca Senior Academic Award;
- 2004, RSC Reaction Mechanism Award;
- 2006–2011Royal Society Wolfson Merit Award;
- 2007, RSC/GDCh–Alexander Todd–Hans Krebs Lectureship;
- 2007, RSC Tilden Lecturer; awarded;
- 2007–2012, EPSRC Senior Research Fellowship;
- 2008, 12th Thieme Lecturer on Organic and Bioorganic Chemistry;
- 2009, RSC Stereochemistry Award;
- 2009, SCI Award for Process Research;
- 2010H. C. Brown Lectureship; Purdue University;
- 2012, Elected Fellow of the Royal Society;
- 2013, Chemical Research Society of India Medal;
- 2013, RSC Perkin Award;
- 2015, Hind Rattan Award;
- 2016, Humboldt Research Award;
- 2019, Arthur C. Cope Scholar Award; American Chemical Society; US;
- 2019, Yamada-Koga Prize
工作介绍
1.锂化-硼化反应
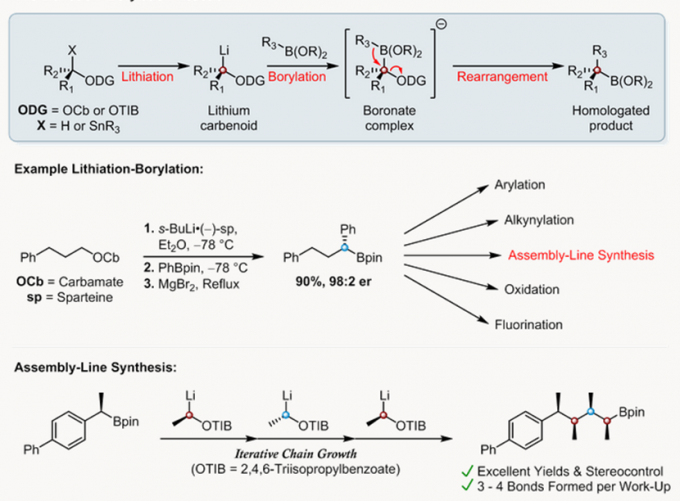
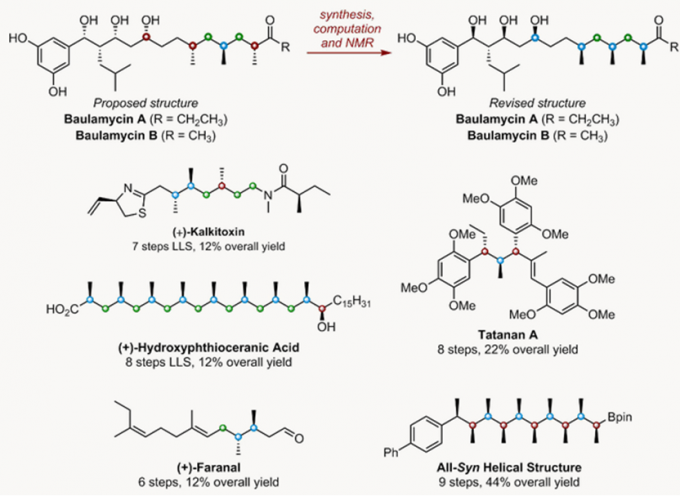
锂化-硼化反应是种可用于合成C-C键的方法,它被Aggarwal教授课题组用来高收率高手性控制的合成烷基硼物种,从而得到传统反应难以得到的复杂结构[1]。这种可用于增碳的有机硼化学有多种用途,以线性组装合成(Assembly-Line Synthesis)为例,在后处理前,4个连续高效的锂化-硼化反应能够得到手性精准控制的多手性中心的产物[2]。
Assembly-Line合成模拟了自然界合成天然产物的方法,精准的控制了分子的立体结构。它在复杂天然产物全合成中有许多应用[3]。
- 无需过渡金属的交叉偶联反应
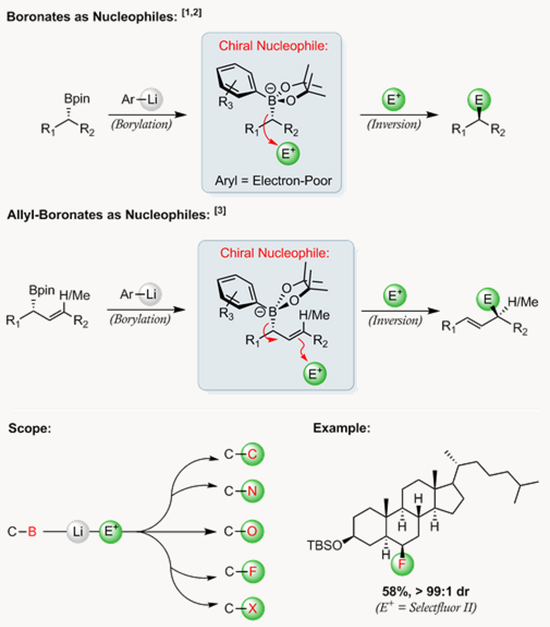
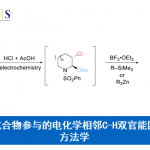
交叉偶联反应在制药工业中占C-C键合成的60%。过渡金属催化的传统偶联反应大都依赖昂贵且部分具有毒性的金属催化剂。因此,Aggarwal教授对开发无需过渡金属的交叉偶联反应具有浓厚的研究兴趣。
根据自己开发的锂化-硼化化学,他们开发了一种无需过渡金属的交叉偶联反应,生成的手性硼酸复合物诱导发生1,2-metallate重排得到偶联产物。这种可靠的方法可用于二级或三级硼酸和一系列芳基发生高对映选择性的偶联反应{4}。
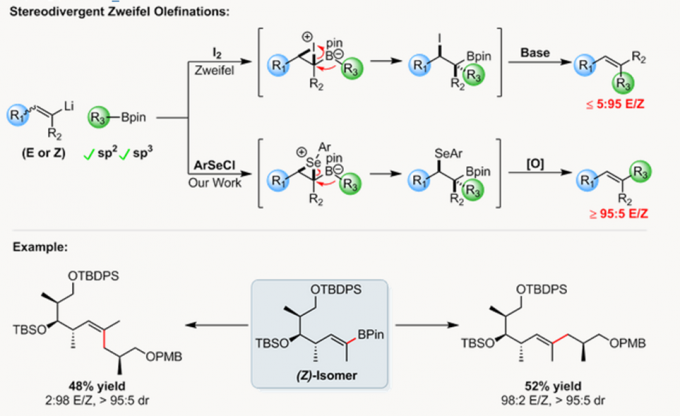
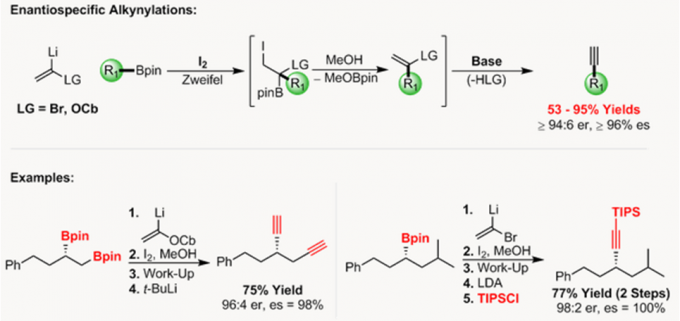
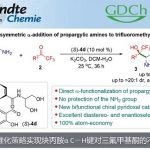
- Zweifel烯基化反应和Enantiospecific炔基化反应[5]
Aggarwal教授参照Zweifel烯基化反应开发了烯基卤化物和硼酸酯间,无需过渡金属的立体选择性偶联反应。此过程可用于sp2和手性sp3硼酸酯的手性保持的偶联反应,并且,该反应可使烯基卤化物选择性的发生反应得到E式或Z式烯烃。
依据Zweifel烯基化反应,Aggarwal教授还开发了对映性保持的脱硼炔基化反应,硼酸酯可为手性二级或三级硼酸酯。
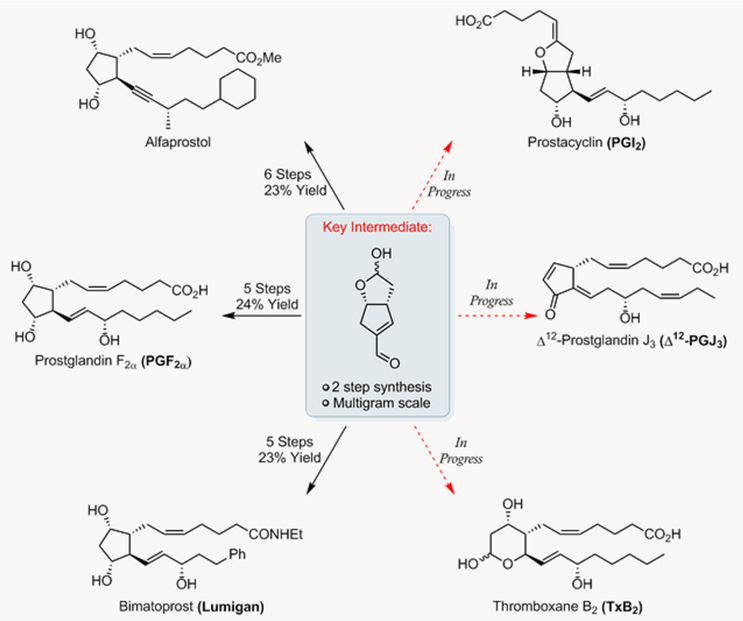
- 前列腺素(Prostanoids)全合成{6}
前列腺素是一类有重要生物功能的天然产物,如血压调控、血小板合成等。自1970年来,吸引了许多合成化学家的兴趣,Aggarwal教授也对前列腺素及其衍生物进行了合成研究。他们通过有机小分子催化丁二醛二聚,立体选择性的合成了一种烯醛高级中间体,此反应可克级规模放大,并且可有效的运用到天然产物的合成中。
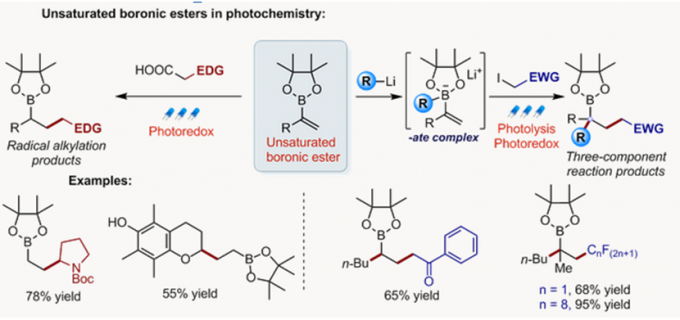
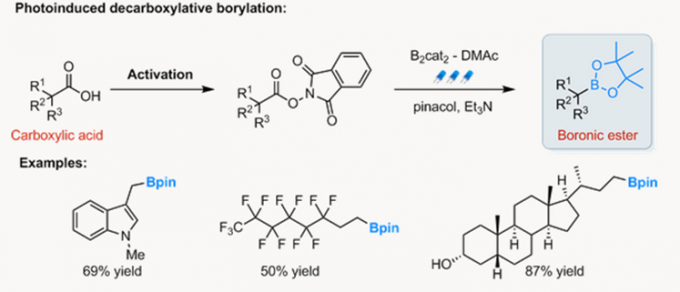
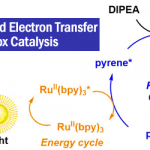
- 光氧化还原反应的开发[7]
光化学在复杂分子化学合成的方法学研究中有重要的作用。Aggarwal教授开发了烯基硼酸酯和烷基自由基结合高收率得到烷基化产物的反应。此外,由硼酸酯与锂试剂反应得到的硼酸复合物与缺电子自由基的多组分反应也得到开发。最近,Aggarwal教授开发了一种将羧酸衍生化得到的高活性物质发生光氧化还原反应后得到多样的硼化产物的方法,该工作发表与2017年science上。
参考文献
1.a: Leonori, D., Aggarwal, V. K., Acc. Chem. Res., 2014, 47, 3174. DOI: 10.1021/ar5002473; b: Essafi, S., Tomasi, S., Aggarwal, V. K., Harvey, J. N., J. Org. Chem., 2014, 79, 12148.DOI: 10.1021/jo502020e; c: Fawcett, A., Nitsch, D., Ali, M., Bateman, J. M., Myers, E. L., Aggarwal, V. K., Angew. Chem. Int. Ed., 2016, 55, 14663. doi.org/10.1002/anie.201608406; d: Varela, A., Garve, L. K. B., Leonori, D., Aggarwal, V. K., Angew. Chem. Int. Ed., 2017, 56, 2127. doi.org/10.1002/anie.201611273;
2: a: Burns, M., Essafi, S., Bame, J. R., Bull, S. P., Webster, M. P., Balieu, S., Dale, J. W., Butts, C. P., Harvey, J. N., Aggarwal, V. K., Nature, 2014, 513, 183. doi.org/10.1038/nature13711; b: Balieu, S., Hallett, G. E., Burns, M., Bootwicha, T., Studley, J., Aggarwal, V. K., J. Am. Chem. Soc., 2015, 137, 4398. DOI: 10.1021/ja512875g;
3: a: Noble, A., Roesner, S., Aggarwal, V. K., Angew. Chem. Int. Ed., 2016, 55, 15920. doi.org/10.1002/anie.201609598; b: Bootwicha, T., Feilner, J. M., Myers, E. L., Aggarwal, V. K., Nature Chem., 2017, 9, 896. doi.org/10.1038/nchem.2757; c:: Wu, J., Lorenzo, P., Zhong, S., Ali, M., Butts, C. P., Myers, E. L., Aggarwal, V. K., Nature, 2017, 547, 436. doi.org/10.1038/nature23265;
4: a: Bonet, A., Odachowski, M., Leonori, D., Essafi, S., Aggarwal, V. K., Nat. Chem., 2014, 6, 584. doi.org/10.1038/nchem.1971; b: Odachowski, M., Bonet, A., Essafi, S., Conti-Ramsden, P., Harvey, J. N., Leonori, D., Aggarwal, V. K., J. Am. Chem. Soc., 2016, 138, 9521. DOI: 10.1021/jacs.6b03963; c: Llaveria J., Leonori D., Aggarwal, V. K., J. Am. Chem. Soc., 2015, 137, 10958. DOI: 10.1021/jacs.5b07842; d: Aichhorn S, Bigler R., Myers E. L., Aggarwal V. K., J. Am. Chem. Soc., 2017, 139, 9519. DOI: 10.1021/jacs.7b05880; e: Ganesh V., Odachowski M., Aggarwal V. K., Angew. Chem. Int. Ed., 2017, 56, 9752. doi.org/10.1002/anie.201703894; f: Wilson C. M., Venkataraman G., Noble A., Aggarwal V. K., Angew. Chem. Int. Ed., 2017, 56, 16318. doi.org/10.1002/anie.201710777;
- a: Armstrong, R. J., García-Ruiz, C., Myers, E. L., Aggarwal, V. K., Angew. Chem. Int. Ed., 2017, 56, 786. doi.org/10.1002/anie.201610387; b: Armstrong, R. J., Niwetmarin, W., Aggarwal, V. K., Org. Lett., 2017, 19, 2762. DOI: 10.1021/acs.orglett.7b01124; c: Wang, Y., Noble, A., Myers, E. L., Aggarwal, V. K., Angew. Chem. Int. Ed., 2016, 55, 4270. doi.org/10.1002/anie.201600599;
6.a: Coulthard, G., Erb, W., Aggarwal, V. K.,Nature, 2012, 489, 278. doi.org/10.1038/nature11411; b: Prévost, S., Thai, K., Schützenmeister, N., Coulthard, G., Erb, W., Aggarwal, V. K., Org. Lett., 2015, 17, 504. DOI: 10.1021/ol503520f; c: H. Baars, M. J. Classen, V. K. Aggarwal, Org. Lett., 2017, 19, 6008. DOI: 10.1021/acs.orglett.7b03057;
- a: Noble, A.; Mega, R. S.; Pflästerer, D.; Myers, E. L.; Aggarwal, V. K.,Angew. Chem. Int. Ed., 2018, 57, 2155. doi.org/10.1002/anie.201712186; b: Silvi, M.; Sandford, C.; Aggarwal, V. K.,J. Am. Chem. Soc.,2017, 139, 5736. DOI: 10.1021/jacs.7b02569; c: Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E. L.; Aggarwal, V. K.,Science2017, 357, 283. DOI: 10.1126/science.aan3679.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!
























No comments yet.