Dean J. Tantillo (1973年xx月xx日-), 是美国计算化学家。加州大学戴维斯分校教授。(写真 http://blueline.ucdavis.edu)
履历
1991-1995 AB degree Harvard University
1995-2000 Ph.D UCLA (Prof. Ken Houk)
2000-2003 postdoctoral Associate Cornell University (Ronald hoffmann)
2003-2008 Assistant Professor UC Davis
2008-2011 Associate Professor UC Davis
2011- Professor UC Davis
获奖经历
- 2015 Academic Senate Distinguished Undergraduate Teaching Award, UC Davis
- 2014 Andrew Streitwieser Lectureship, UC Berkeley
- 2014 Soaring to New Heights Diversity and Principles of Community Faculty Diversity Award, UC Davis
- 2012 Natural Product Reports Lectureship
- 2014 Andrew Streitwieser Lectureship, UC Berkeley
- 2014 Soaring to New Heights Diversity and Principles of Community Faculty Diversity Award, UC Davis
- 2012 Natural Product Reports Lectureship
- 2011 National Academy of Sciences Kavli Fellow
- 2011 Lawrence J. Schaad Lectureship in Theoretical Chemistry, Vanderbilt University
- 2011 Academic Senate Distinguished Undergraduate Teaching Award, UC Davis
- 2010 National Academy of Sciences Kavli Fellow
- 2007 Journal of Physical Organic Chemistry Award for Early Excellence in the Field of Physical Organic Chemistry
- 2005 National Science Foundation CAREER Award
- 2000 Winstein Award for Outstanding Dissertation Research in Organic Chemistry, Department of Chemistry and Biochemistry, UCLA
- 2000 Distinguished Teaching Assistant Award, Academic Senate Committee on Teaching, UCLA
- 2000 First Prize for Graduate Students, 52nd Annual Robert B. and Blanche Campbell Student Book Collection Competition, UCLA Library
- 1999 Jacobs Award for Outstanding Research, Department of Chemistry and Biochemistry, UCLA
- 1999 Departmental Prize for Distinguished Teaching in Chemistry and Biochemistry, UCLA
研究
计算化学大牛Kendall Houk实验室获得的博士学位。当时,其博士的研究内容是使用QM/MM进行酶促反应的解析。自己独立后,主要集中于小分子化合物的计算。特别是关于天然产物的生物合成反应机理的理论计算。
- 着眼于碳阳离子的萜烯生物合成计算
萜烯是由甲羟戊酸途径产生的具有异戊二烯C5结构单元的化合物。虽然GPP、FPP、GGDP等起始原料不同,然而它们进行的生物合成路径有一个共同点,都是以环化酶的活性中心存在的Mg进行二磷酸消除开始的反应。二磷酸的消除产生的碳正离子的稳定化过程是反应进行的驱动力。
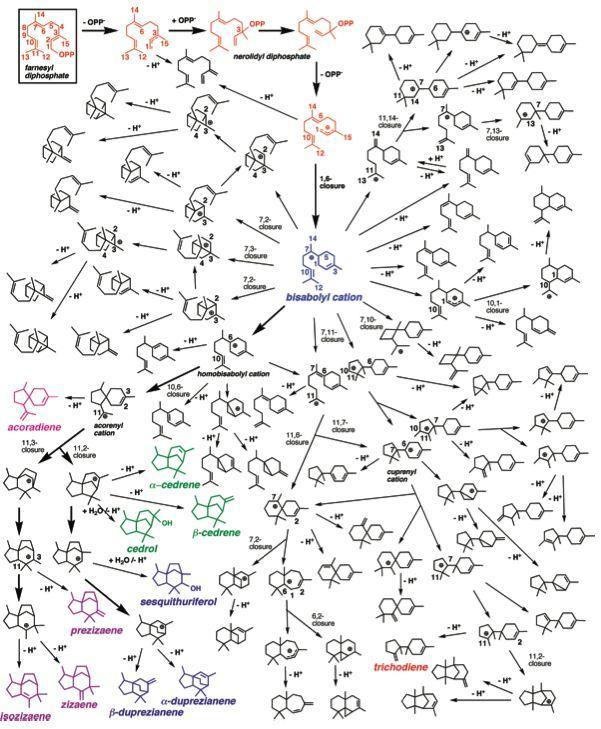
(图引用自文献[1])
而且,在萜烯的环化反应中,经常发生氢转移。Tantillo等人的理论计算结果表明其实这不是氢转移,实际上是质子转移[2]。
另外,虽然广泛认为反应的过渡态,反应中间体的碳正离子为3级碳正离子,而报道表明实际上是离域形式的非典型的碳正离子存在。
相关文献
- Hong, Y. J.; Tantillo, D. J. J. Am. Chem. Soc. 2009, 131, 7999. DOI: 10.1021/ja9005332
- Hong, Y. J.; Tantillo, D. J. J. Am. Chem. Soc. 2015, 137, 4134. DOI: 10.1021/ja512685x
- Hong, Y. J.; Giner, J. L.; Tantillo, D. J. J. Am. Chem. Soc. 2015, 137, 2085. DOI: 10.1021/ja512901a
外部链接
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!





















No comments yet.