概要
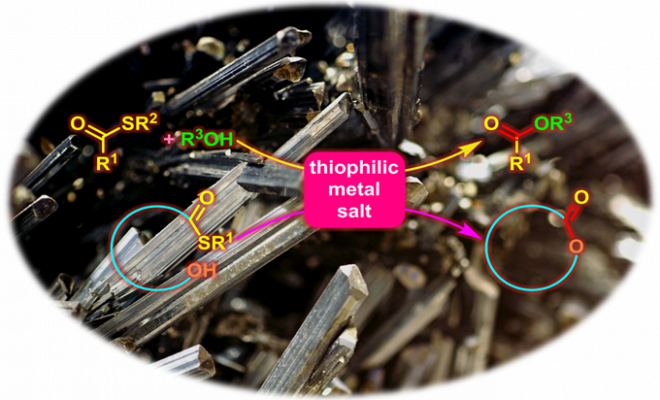
Masamune 反应 (Masamune reaction)是在亲硫性金属盐 (thiophilic metal salt),即汞盐 (如三氟乙酸汞[1])、银盐[2] (如三氟乙酸银)、铜盐[2] (如三氟甲磺酸铜)或亚铜盐[2] (如三氟乙酸亚铜或三氟甲磺酸亚铜)存在下,硫代羧酸酯基团与醇羟基官能团之间进行的分子内或分子间酯化过程,形成酯或大环内酯 (形成大环内酯的反应文献中称为Masamune大环内酯化,Masamune macrolactonization)的反应。该反应由加拿大Alberra大学化学系 (Department of Chemistry, University of Alberra)的Masamune (正宗 悟, Masamune Satoru)研究室在1975年首次报道[1]。
Masamune反应条件温和,具有优良的产率与良好的底物适用范围。目前该反应已经广泛应用于各类生物活性天然产物全合成的关键步骤[2]-[17]。
基本文献
- [1] S. Masamune, S. Kamata, W. Schilling, J. Am. Chem. Soc. 1975, 97, 3515. doi: 10.1021/ja00845a039.
- [2] S. Masamune, Y. Hayase, W. Schilling, W. Chan, G.S. Bates, J. Am. Chern. Soc. 1977, 99, 6756. doi: 10.1021/ja00462a049.
- [3] S. Masamune, G. S. Bates, J. W. Corcoran, Angew. Chem. Int. Ed. 1977, 16, 585. doi: 10.1002/anie.197705851.
- [4] S. Masamune, H. Yamamoto, S. Kamata, A. Fukuzawa, J. Am. Chem. Soc. 1975, 97, 3513. doi: 10.1021/ja00845a038.
- [5] S. Masamune, M. Hirama, S. Mori, S. A. Ali, D. S. Garvey, J. Am. Chem. Soc. 1981, 103, 1568. doi: 10.1021/ja00396a051.
- [6] C. M. J. Fox, S. V. Ley, A. M. Z. Slawin, D. J. Williams, J. Chem. Soc., Chem. Commun. 1985, 1805. doi: 10.1039/C39850001805.
- [7] P. M. Booth, H. B. Broughton, M. J. Ford, C. M. J. Fox, S. V. Ley, A. M. Z. Slawin, D. J.Williams, P. R.Woodward, Tetrahedron 1989, 45, 7565. doi: 10.1016/S0040-4020(01)89218-8.
- [8] S. V. Ley, P. R. Woodward, Tetrahedron Lett. 1987, 28, 345. doi: 10.1016/S0040-4039(00)95725-3.
- [9] P. U. Park, C. A. Broka, B. F. Johnson, Y. Kishi, J. Am. Chem. Soc. 1987, 109, 6205. doi: 10.1021/ja00254a062.
- [10] H. Toshima, T. Suzuki, S. Nishiyama, S. Yamamura, Tetrahedron Lett. 1989, 30, 6725. doi: 10.1016/S0040-4039(00)70661-7.
- [11] K. Tatsuta, A. Nakagawa, S. Maniwa, M. Kinoshita, Tetrahedron Lett. 1980, 21, 1479. doi: 10.1016/S0040-4039(00)92751-5.
- [12] R. E. Ireland, M. D. Varney, J. Org. Chem. 1986, 51, 635. doi: 10.1021/jo00355a013.
- [13] J. Huang, J. Meinwald, J. Am. Chem. Soc. 1981, 103, 861. doi: 10.1021/ja00394a022.
- [14] S. Masamune, L. D. L. Lu, W. P. Jackson, T. Kaiho, T. Toyoda, J. Am. Chem. Soc. 1982, 104, 5523. doi: 10.1021/ja00384a061.
- [15] B. M. Trost, P. G. McDougal, J. Org. Chem. 1984, 49, 458. doi: 10.1021/jo00177a015.
- [16] A. Parenty, X. Moreau, J.-M. Campagne, Chem. Rev. 2006, 106, 911. doi: 10.1021/cr0301402.
- [17] A. Parenty, X. Moreau, G. Niel, J.-M. Campagne, Chem. Rev. 2013, 113, 1, PR1. doi: 10.1021/cr300129n.
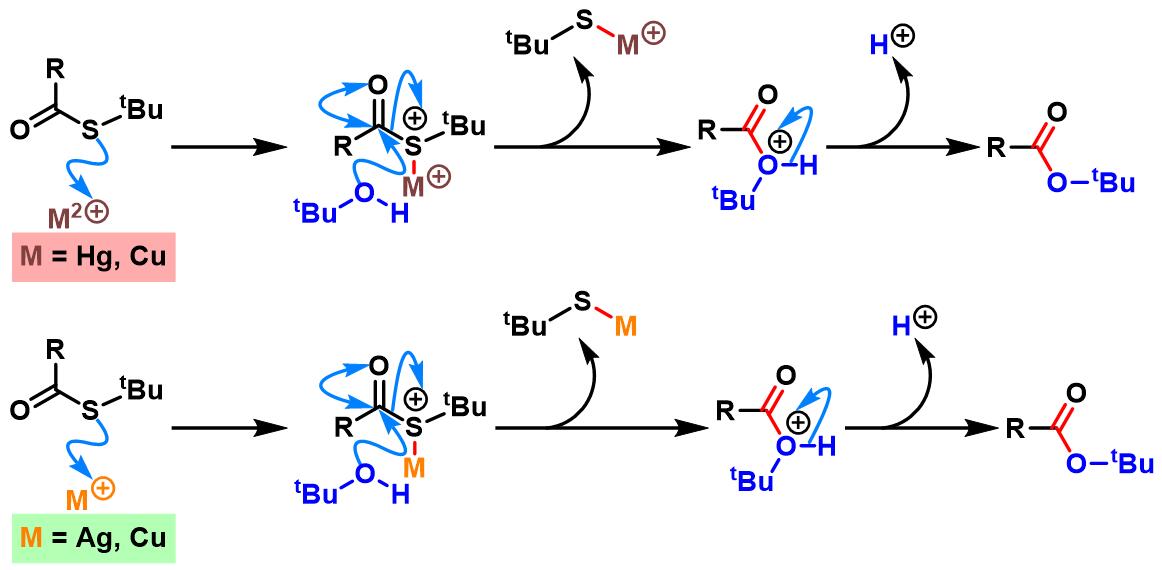
反应机理
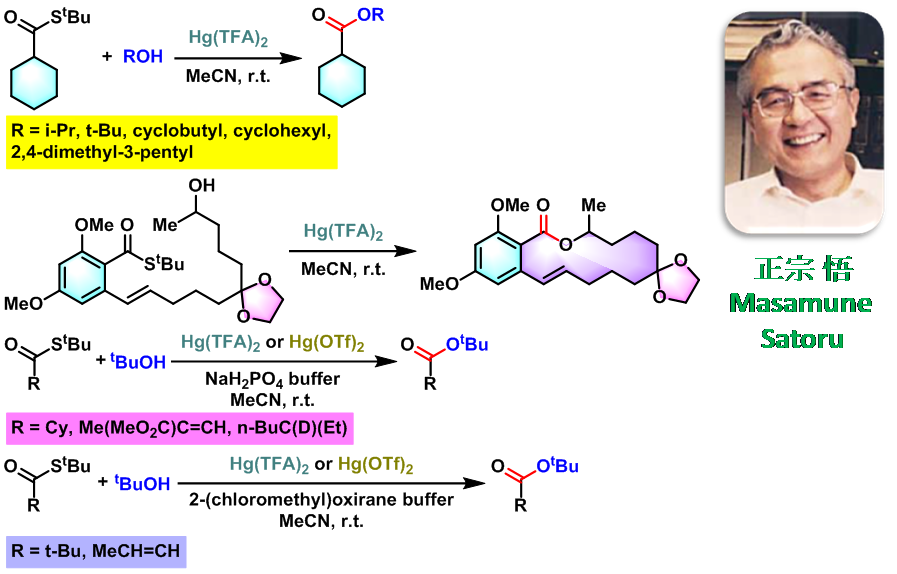
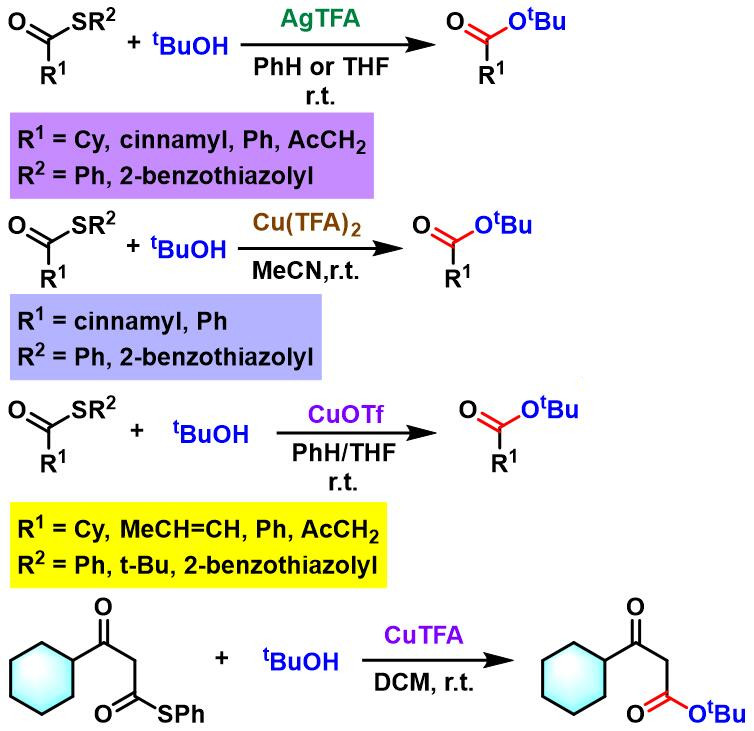
反应实例
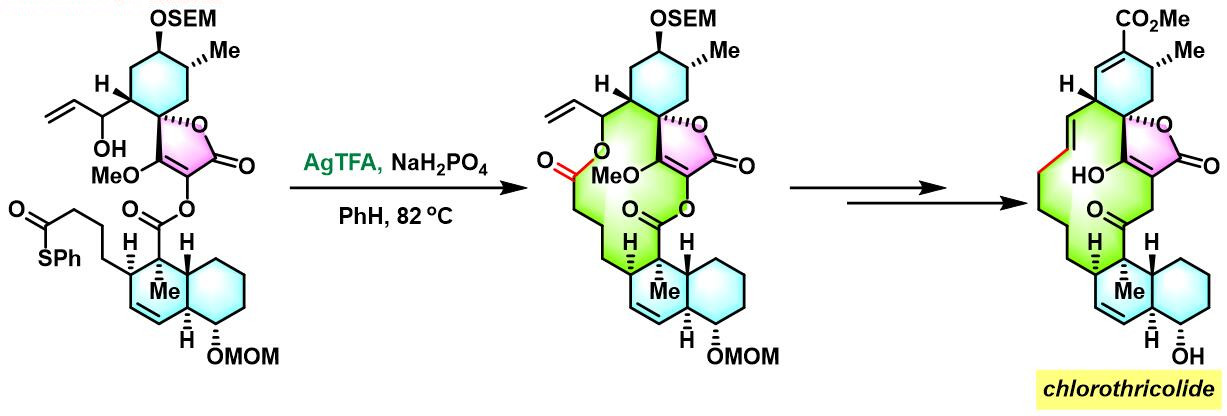
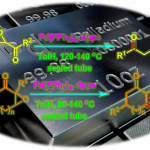
chlorothricolide的全合成[1]
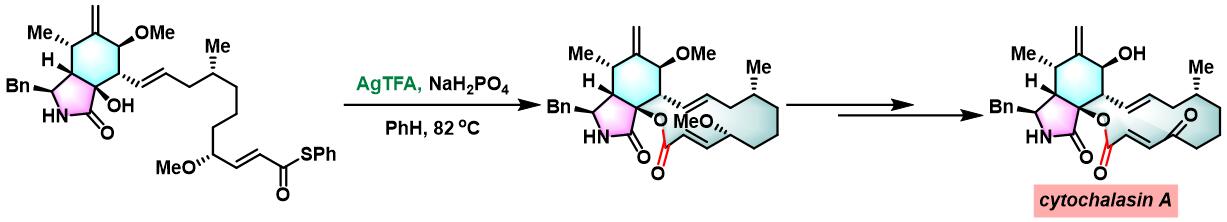
cytochalasin A的全合成[2]
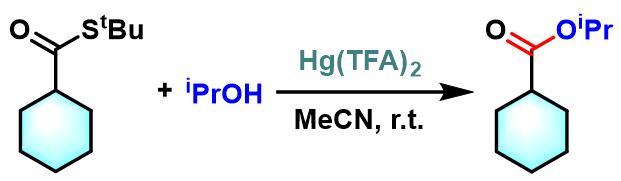
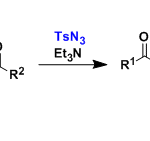
酯的合成[3]
实验步骤
参考文献
- [1] R. E. Ireland, M. D. Varney, J. Org. Chem. 1986, 51, 635. doi: 10.1021/jo00355a013.
- [2] S. Masamune, Y. Hayase, W. Schilling, W. Chan, G.S. Bates, J. Am. Chem. Soc. 1977, 99, 6756. doi: 10.1021/ja00462a049.
- [3] S. Masamune, S. Kamata, W. Schilling, J. Am. Chem. Soc. 1975, 97, 3515. doi: 10.1021/ja00845a039.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!
















No comments yet.