本文作者:石油醚
概要
Svetlana B. Tsogoeva, 德国埃尔朗根-纽伦堡大学有机化学系教授,有机化学家。主要专注于不对称有机催化以及合成化合物的活性研究。
课题组主页:https://www.chemistry.nat.fau.eu/tsogoeva-group/
经历
- 1990年07月-1995年06月 圣彼得堡国立大学化学系获得学士学位
- 1995年06月-1998年10月 圣彼得堡国立大学化学系获得博士学位
- 1998年11月-2000年06月 法兰克福大学博士后研究
- 2000年07月-2001年12月 Degussa AG and Creavis GmbH精细化学部研究员
- 2002年01月-2007年01月 哥廷根大学初级教授
- 2007年02月-至今 埃尔朗根-纽伦堡大学有机化学系教授
获奖经历
- 2012: Otto-Röhm Research Award
- 2008: Tetrahedron: Asymmetry “Most Cited Paper 2005-2008 Award”
- 2007: Thieme Chemistry Journal Award 2007
- 2006: CERC3 (Chairmen of the European Research Councils’s Chemistry Committees) Lecture Travel Award
- 2006: GDCh (Gesellschaft Deutscher Chemiker) Lecture Travel Award
- 2006: FCI (Fonds der Chemischen Industrie) Lecture Travel Award
- 2005: JSP Junior Scientist Award (EUCHEM)
- 2003: Research Award from the Fonds der Chemischen Industrie (FCI)
- 1998-2000: DFG Postdoctoral Research Fellowship
- 1995-1998: Procter & Gamble Postgraduate Research Fellowship
研究方向
1. 不对称催化
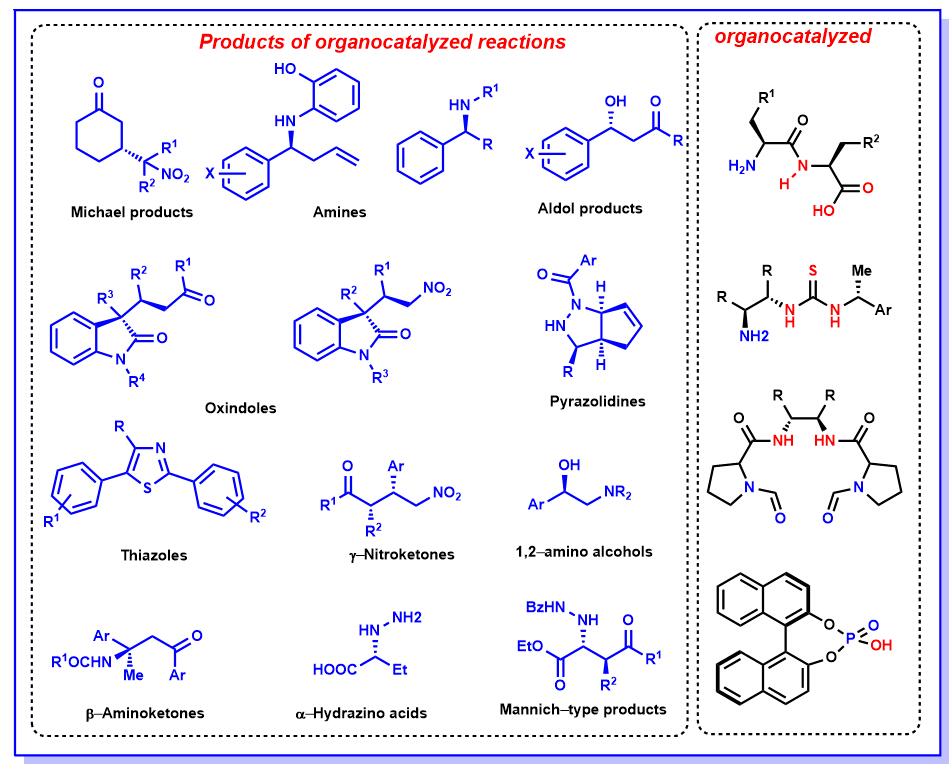
(1)开发原料易于获取且环境友好的一锅合成(如多米诺反应)并将其应用于具有生物活性杂环等有机分子的合成中。通过减少后处理步骤和反应阶段,极大的节省实验室工作时间,并可以降低合成的成本。Svetlana B. Tsogoeva , 小组目前的针对性开展这种环境友好和经济高效的方法的研究1-5。
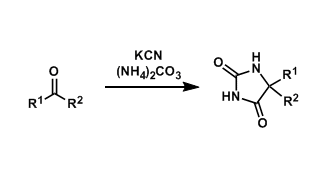
(2)新型手性双官能团化催化剂的开发以及在常规溶剂和水中的反应尝试6-12。(Fig.1)
Figure 1 手性催化剂
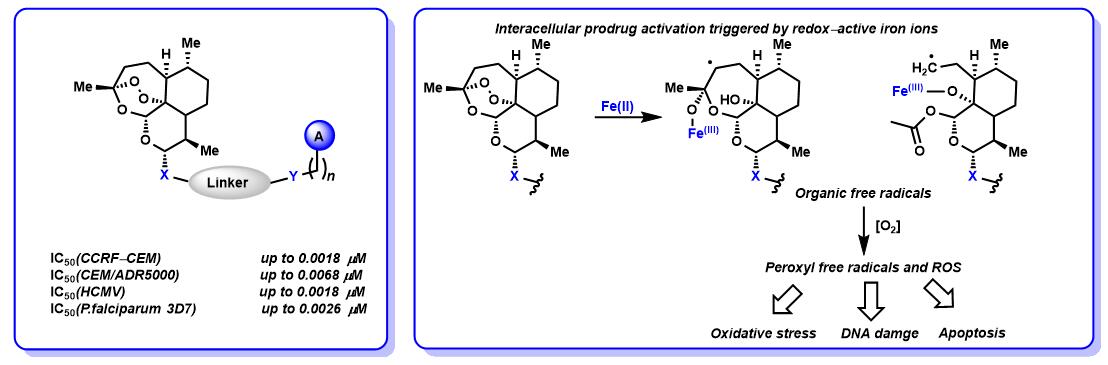
2. 杂合分子的活性研究
癌症治疗以及使用多种耐药菌株治疗病毒和疟疾感染是现代医学面临的最大挑战之一。因此,有机和药物化学的一个重要目标是针对性的开发治疗疾病的新型药理活性药物分子。一种新的有前途的方法是开发基于已知天然产物和天然产物类似物的杂合分子。这种方法对于开发用于医学应用新的先导结构非常有吸引力,因为这些杂合分子的生物学活性在许多情况下都超过了相应母体化合物的生物学活性。已经显示出,杂合分子与其抗病毒,多抗性疟疾株和抗性癌细胞的单个组分相比具有更高的活性。青蒿琥酯是青蒿素的半合成衍生物,青蒿素是一种在癌细胞中显示出细胞毒活性的药物。我们目前正在合成含青蒿琥酯和其他具有生物活性的杂合分子,用于抗癌,抗病毒和抗疟疾研究5,13-20。(Fig.2)
Fig.2 杂合分子的活性研究
3. 通过结晶对手性化合物进行连续拆分
其他
课题组照片
参考文献
- [1] Wei, S., Messerer, R. & Tsogoeva, S. B. Asymmetric Synthesis of β-Adrenergic Blockers through Multistep One-Pot Transformations Involving In Situ Chiral Organocatalyst Formation. Chem. Eur. J. (2011) 17, 14380-14384, doi:10.1002/chem.201102931.
- [2] Weiß, K. M., Wei, S. & Tsogoeva, S. B. Novel one-pot process for the synthesis of 1,3-thiazolesvia organocatalysed epoxidation of nitro-olefins. Org. & Bio. Chem. (2011) 9, 3457-3461, doi:10.1039/C1OB05260H.
- [3] Held, F. E. et al. Facile access to potent antiviral quinazoline heterocycles with fluorescence properties via merging metal-free domino reactions. Nat. Commun. (2017) 8, 15071, doi:10.1038/ncomms15071.
- [4] Belletti, G. et al. Photoracemization-Based Viedma Ripening of a BINOL Derivative. Chem. Eur. J. (2020) 26, 839-844, doi:10.1002/chem.201904382.
- [5] Çapcı, A. et al. Artemisinin–(Iso)quinoline Hybrids by C−H Activation and Click Chemistry: Combating Multidrug-Resistant Malaria. Angew. Chem. Int. Ed. (2019) 58, 13066-13079, doi:10.1002/anie.201907224.
- [6] Bock, C. M. et al. Generation of Complex Azabicycles and Carbobicycles from Two Simple Compounds in a Single Operation through a Metal-Free Six-Step Domino Reaction. Chem. Eur. J. (2016) 22, 5189-5197, doi:10.1002/chem.201504798.
- [7] Reiter, C. et al. Michael Addition of N-Unprotected 2-Oxindoles to Nitrostyrene Catalyzed by Bifunctional Tertiary Amines: Crucial Role of Dispersion Interactions. ChemCatChem (2014) 6, 1324-1332, doi:10.1002/cctc.201301052.
- [8] Held, F. E., Wei, S., Eder, K. & Tsogoeva, S. B. One-pot route to β-adrenergic blockers via enantioselective organocatalysed epoxidation of terminal alkenes as a key step. RSC Advances (2014) 4, 32796-32801, doi:10.1039/C4RA04011B.
- [9] Tsogoeva, S. B. Transition Metals for Organic Synthesis. Building Blocks and Fine Chemicals. Edited by Matthias Beller and Carsten Bolm. Angew. Chem. Int. Ed. (2005) 44, 3337-3338, doi:10.1002/anie.200485249.
- [10] Mauksch, M., Tsogoeva, S. B., Martynova, I. M. & Wei, S. Evidence of Asymmetric Autocatalysis in Organocatalytic Reactions. Angew. Chem. Int. Ed. (2007) 46, 393-396, doi:10.1002/anie.200603517.
- [11] Yalalov, D. A., Tsogoeva, S. B., Shubina, T. E., Martynova, I. M. & Clark, T. Evidence for an Enol Mechanism in a Highly Enantioselective Mannich-Type Reaction Catalyzed by Primary Amine–Thiourea. Angew. Chem. Int. Ed. (2008) 47, 6624-6628, doi:10.1002/anie.200800849.
- [12] Tsogoeva, S. B., Wei, S., Freund, M. & Mauksch, M. Generation of Highly Enantioenriched Crystalline Products in Reversible Asymmetric Reactions with Racemic or Achiral Catalysts. Angew. Chem. Int. Ed. (2009) 48, 590-594, doi:10.1002/anie.200803877.
- [13] Fröhlich, T., Çapcı Karagöz, A., Reiter, C. & Tsogoeva, S. B. Artemisinin-Derived Dimers: Potent Antimalarial and Anticancer Agents. J. Med. Chem. (2016) 59, 7360-7388, doi:10.1021/acs.jmedchem.5b01380.
- [14] Reiter, C. et al. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorg. Med. Chem. 2015, 23, 5452-5458. (2015) 23, 5452-5458, doi:https://doi.org/10.1016/j.bmc.2015.07.048.
- [15] Reiter, C. et al. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur. J. Med. Chem. (2015) 97, 164-172, doi:https://doi.org/10.1016/j.ejmech.2015.04.053.
- [16] Reiter, C. et al. Synthesis and study of cytotoxic activity of 1,2,4-trioxane- and egonol-derived hybrid molecules against Plasmodium falciparum and multidrug-resistant human leukemia cells. Eur. J. Med. Chem. (2014) 75, 403-412, doi:https://doi.org/10.1016/j.ejmech.2014.01.043.
- [17] Jacquet, C. et al. A highly potent trimeric derivative of artesunate shows promising treatment profiles in experimental models for congenital HCMV infection in vitro and ex vivo. Antiviral Res. (2020) 175, 104700, doi:https://doi.org/10.1016/j.antiviral.2019.104700.
- [18] Karagöz, A. Ç. et al. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2015, 23, 5452-5458. (2019) 27, 110-115, doi:https://doi.org/10.1016/j.bmc.2018.11.018.
- [19] Sonntag, E. et al. In vivo proof-of-concept for two experimental antiviral drugs, both directed to cellular targets, using a murine cytomegalovirus model. Antiviral Res. (2019) 161, 63-69, doi:https://doi.org/10.1016/j.antiviral.2018.11.008.
- [20] Çapcı Karagöz, A. et al. Access to new highly potent antileukemia, antiviral and antimalarial agents via hybridization of natural products (homo)egonol, thymoquinone and artemisinin. Bioorg. Med. Chem. 2015, 23, 5452-5458. (2018) 26, 3610-3618, doi:https://doi.org/10.1016/j.bmc.2018.05.041.
本文版权属于 Chem-Station化学空间, 欢迎点击按钮分享,未经许可,谢绝转载!






















No comments yet.